Question
Question: : How does benzene differ from other cyclic hydrocarbons?...
: How does benzene differ from other cyclic hydrocarbons?
Solution
Aromatic compounds and the compounds that possess an alternate double and single bond system, where delocalization of electrons can occur.
Complete step-by-step answer: The compound with the formulaC6H6, contains an alignment with alternate single and double bond inside as ring structure. This compound is known as benzene. Benzene has an aromatic nature, this is proven by the fact that, when we burn it or does a flame test, it burns with a smoky flame; this is the property of aromatic compounds. While, aliphatic compounds burns with flame that is does not produce smoke. Also due to its high carbon content, it has sooty deposits while burning.
Benzene has a cyclic and a planar structure with a six-member carbon ring. The structure of benzene allows delocalization of electrons. The structure is as follows:
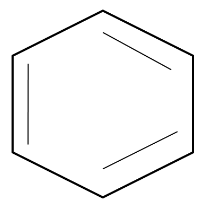
As benzene allows complete delocalization of electrons (pi-electrons), it has conjugation that allows it to participate in electrophilic substitution reactions. This property is possessed by benzene and not by other cyclic hydrocarbons due to absence of aromaticity.
The electrophilic substitution reactions of benzene occur as when an electrophile attacks the benzene, hydrogen gets removed, in the intermediate step, the benzene cation formed by removal of hydrogen is highly reactive, that allows electrophilic substitution on the place of removal of hydrogen. Nitration, sulphonation, Friedal-crafts reactions, are some of the examples of electrophilic substitution reactions of benzene.
Hence, benzene differs in aromaticity and electrophilic substitution reactions from other cyclic hydrocarbons.
Note: Benzene has all the carbons as sp2 hybridized. All the bond lengths are equal in benzene due to resonance, this makes it highly stable and easily available for electrophilic substitution reaction.
