Question
Question: How do you write the orbital diagram for phosphate?...
How do you write the orbital diagram for phosphate?
Solution
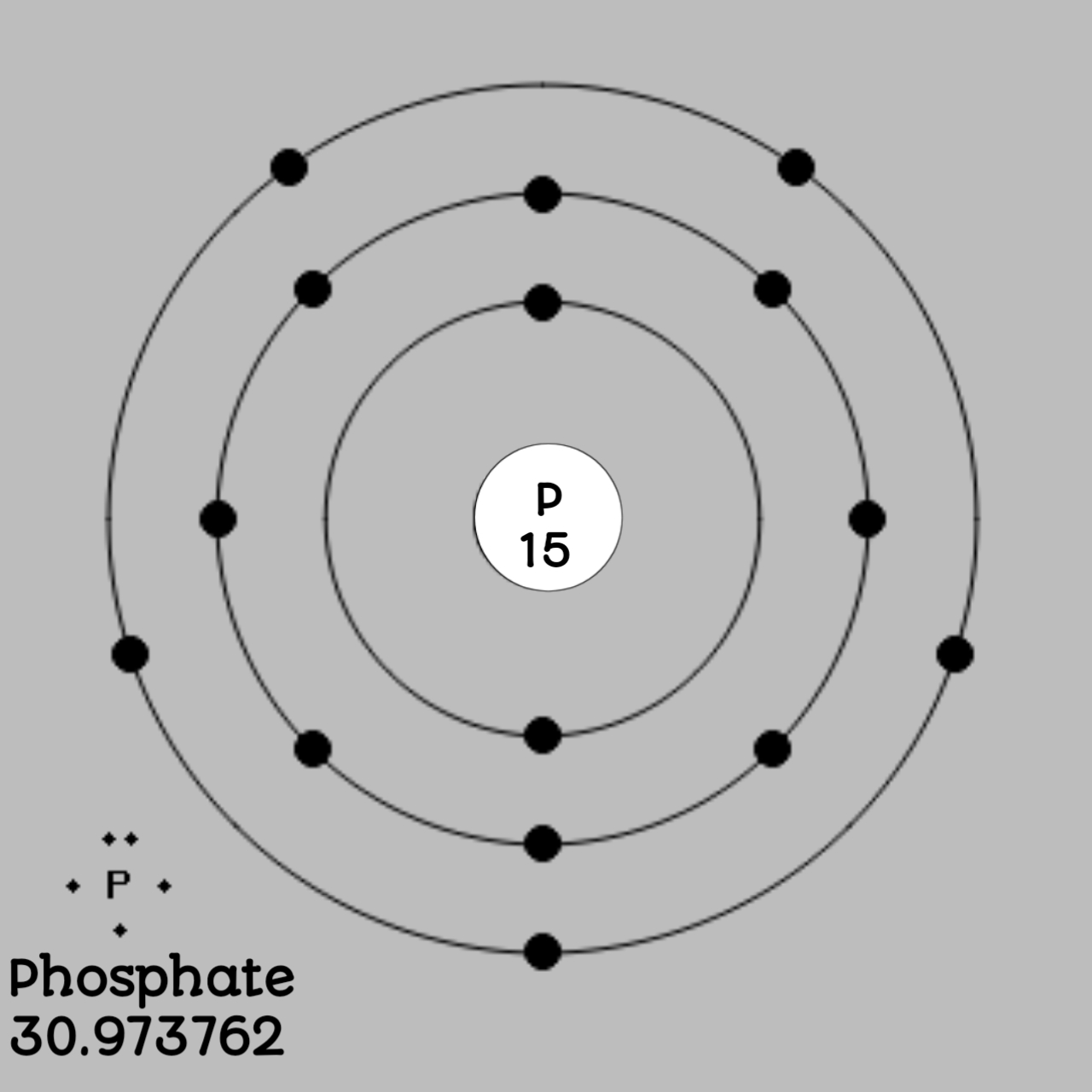
Phosphorus has an atomic number 15 . As the number of protons in sulfur is 15 there are 15 electrons in orbit around the nucleus.
Also Electrons always revolve around the nucleus of an atom and are found in shells which consist of sub shells. The nth shell here can have a total up to 2n2 electron. Each shell is made up of sub shells and a shell with nay value of n can have n number of sub shells that have are given a value l = 0, 1, 2, 3………... (n − 1) with respect to s, p, d, f subshells. For any value of l, the subshell can have 2(2l + 1) electrons.
Complete answer:
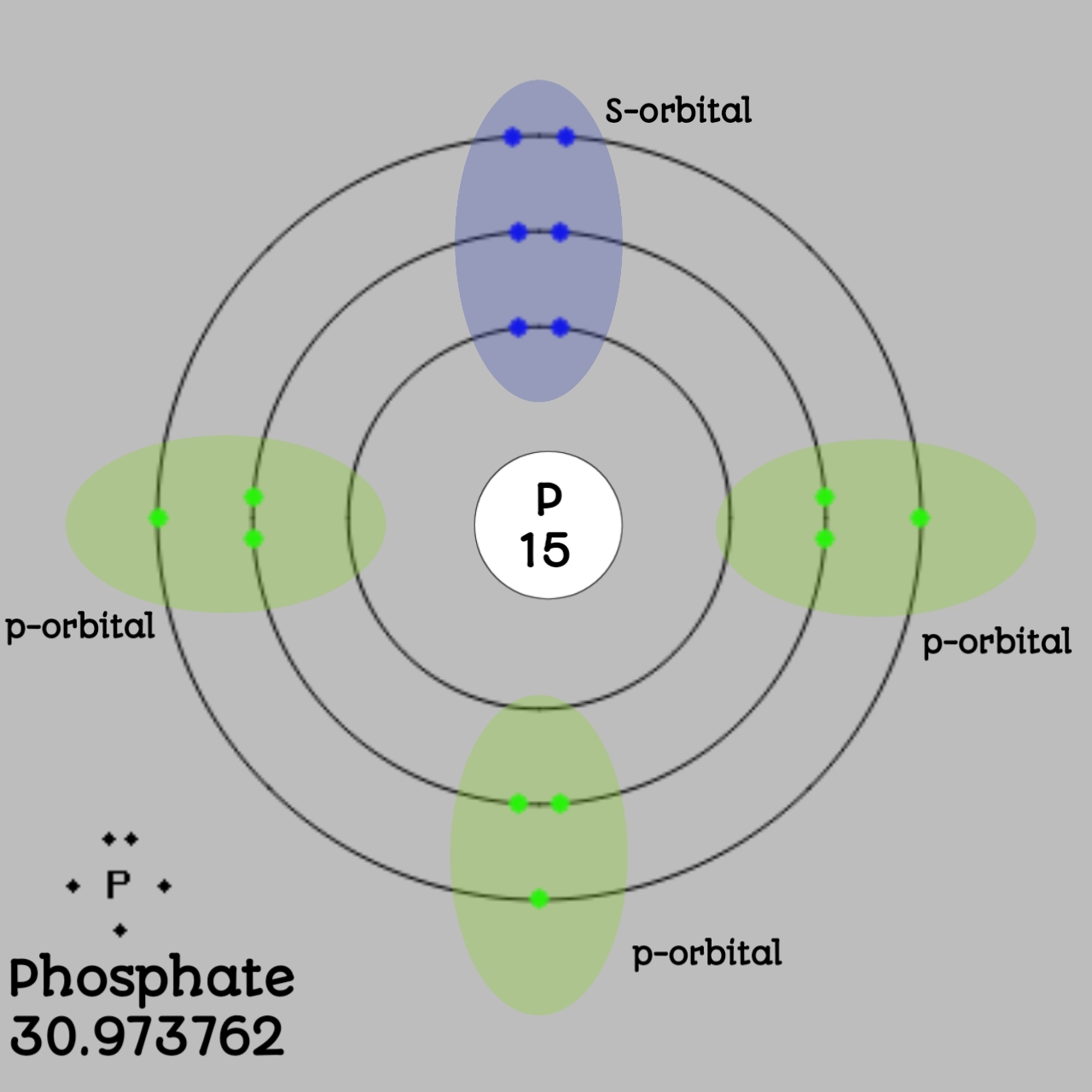
We all know that the first shell have only one sub shell which has 1 value of 0 and that is s subshell.
Likewise the second shell has 2 sub shells, s and p and can easily accomplish 8 electrons and the third subshell has 3 subshells that are s, p and d.
For phosphate, the first shell has 2 electrons, the second shell has 8 electrons and the third shell has 5 electrons.
The electron configuration of phosphate can be given as 1s2 2s2 2p6 3s2 3p3
The noble gas has a configuration that is the having equal configuration of the electrons in an excess to the number of electrons in a noble gas with atomic number that is just less than element. For phosphate this gas is Neon gas.
The whole point of these was to analyse how the oxygen orbital energies split up which were two fold or threefold degenerate itself. It was also to analyse which orbital on phosphate interacts with which of orbitals in the oxygen atom.
The resultant MO diagram of phosphate is given by:
Note: -Note that this is only qualitative, so take it with a grain of salt.
Similarly Phosphate has all electrons paired.
-The nonbonding orbitals are nonbonding either due to mismatched symmetries, overly different inner-atom outer-atom orbital energies, or interactions between both high-lying and low-lying orbitals.
-There are 22 non bonding electrons, which accounts for all 11 lone pairs of electrons that belong to the oxygen atoms. The remaining 2 nonbonding electrons account for the of π bond that is delocalized
-The σ bond order that this MO diagram seems to account for is 1 for all four σbonds, as there are four clearly-bonding MOs ( a1 + t2 )one for each bond.
