Question
Question: How do you write the noble-gas electron configuration for copper?...
How do you write the noble-gas electron configuration for copper?
Solution
The electronic configuration says about how the electrons are distributed in atomic orbitals. The electronic configuration is filled according to the energy they have. They follow the standard notion in which electrons containing the atomic subshells are placed in a sequence. For example, the Copper’s electronic configuration; Cu=1s22s22p63s23p64s13d10.
Complete step-by-step answer:
The copper is the 29th element in the periodic table. Its electronic configuration is as follows;
Cu= 1s22s22p63s23p64s13d10
Whereas, in the condensed form of electronic configuration, the noble gas makes it easier to denote it.
So, let’s see how the noble gas configuration of copper will be;
Cu= [Ar]4s13d10
Explanation:
Copper (Cu) whose atomic number is 29 , which is having 29 electrons. The 29 protons are there in atomic nuclei. A neutral atom has the same number of protons as well as the same number of electrons.
When we write the condensed form or abbreviated form of electronic configuration of copper, we have to start writing the noble gas configuration in the previous period, and then continue with the electronic configuration of the element in the new period. The noble gas symbol is enclosed in brackets. Then the next periods’ electronic configuration is added.
When it comes to the case of copper, it is in the 4th period of the table, so that the noble gas will be argon, which is the noble gas in period 3 . Since the copper is in the group 11 and the electronic configuration will be ending with 3d and 4s sublevels.
Additional information:
The full electronic configuration of any element is written in the standard format. But when it comes to higher elements will consist of many numbers of orbital, so for simplification condensed electronic configuration is noted.
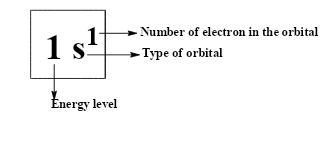
Suppose if we have considered iron as an example,
The full electronic configuration: Fe= 1s22s22p63s23p64s23d6
The condensed electron configuration:Fe= [Ar]4s23d6
Note: The standard notation yields lengthy electronic configuration (which means the electronic configuration will be written as, (Na= 1s22s22p63s1) . In those cases, condensed or an abbreviated notation is used instead of using standard electronic configuration. In condensed electron configuration, the sequence of filled subshell corresponds to the electronic configuration of a noble gas or inert gas is replaced with the symbol of that noble gas in a square bracket, Na= [Ne]3s1 .
