Question
Question: How do you write the formula of periodic acid?...
How do you write the formula of periodic acid?
Explanation
Solution
Periodic acid is the oxoacid of the iodine. The iodine in the periodic acid has an oxidation state + 7 and consists of four oxygen atoms in its structure. One can try to predict the structure and molecular formula of periodic acid based on this information.
Complete step by step answer:
- First of all, we will understand the chemical compound periodic acid. Periodic acid consists of iodine and it is the highest oxoacid of iodine. The oxoacid can be defined as the element which has oxygen, hydrogen, and another element in it.
- Now as the periodic acid is an oxoacid of iodine, the element iodine has an + 7 oxidation state in it which means there are three oxygen atoms that are double bonded to the central iodine atom and one hydroxyl group which is single bonded to the central atom.
- Now let us see the structure of periodic acid as below,
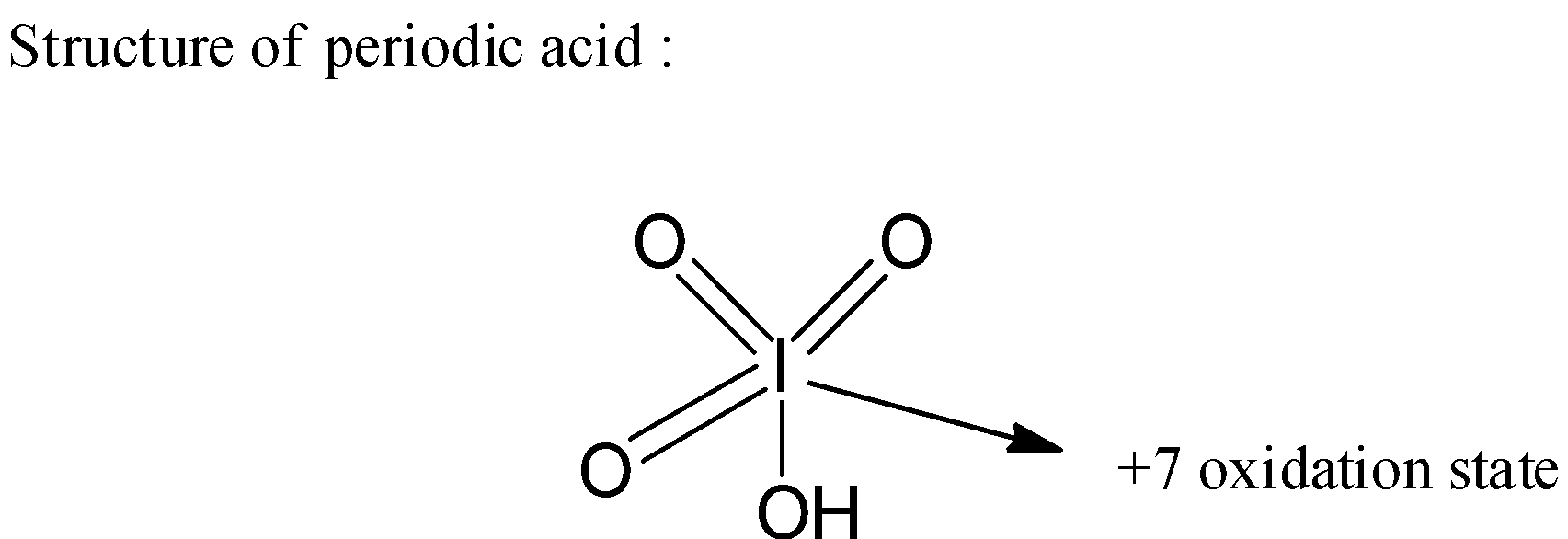
- Now if we observe the above structure the + 7 oxidation state of the central iodine atom is due to surrounding three double bonded oxygens which has - 2 oxidation state each and one - OH group which has an oxidation state as - 1.
Therefore, we can write the formula of the periodic acid as HIO4.
Note:
The periodic acid is a periodate that means the structure can exist in two forms such as orthoperiodic acid which has a chemical formula as H5IO6 and meta periodic acid which has a chemical formula as HIO4. Periodic acid is soluble in water and alcohols and has a melting point of 128⋅5oC.
