Question
Question: How do you write electronic configuration for oxygen using Hund’s rule and Pauli’s exclusion princip...
How do you write electronic configuration for oxygen using Hund’s rule and Pauli’s exclusion principle?
Solution
Atom is the smallest unit of matter. Atom is built by filling the electrons in various orbitals. In chemistry we have various rules which determines how electrons are arranged in an atom. These rules should be followed while writing electronic configuration of any element.
Complete step-by-step answer: In atomic chemistry, we know that certain set of rules that one should follow while writing the electronic configuration. Two of them are Hund’s rule and Pauli’s exclusion principle. Let us see firstly what these rules are.
Hund’s rule of maximum multiplicity: This rule deals with filling of electrons in equal energy orbitals of the same subshell. It states that no pairing of electrons starts in any of the degenerate orbitals until all the orbitals of the subshell contain one electron each with parallel spin.
Pauli’s exclusion principle: It states that no two electrons in an atom can have the same set of four quantum numbers. Even if the three quantum numbers n l m are the same the fourth spin quantum number will always be different.
Now, in question we are asked to write down the electronic configuration based on the above two rules.
We know that Oxygen has its electrons filled over 1s,2s,2porbitals.
Now let’s fill the electrons in them as per the above rules. Let us represent these orbitals with boxes.
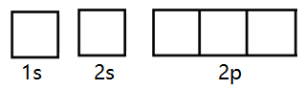
We know that there are 8electrons in oxygen. So, we will start filling them orbital by orbital.
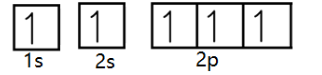
Here we observe that out of 8,5electrons are filled and 3 more needs to be filled. While filling we didn’t fill two electrons in 1s as shown above, as it would have violated Hund’s rule. We firstly filled all orbitals with one electron and now will start pairing.
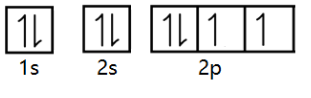
Now, we have finally filled all the electrons. Also note that in the above arrangement of electrons, electrons in each orbital have opposite spin (represented by the opposite arrows). This signifies that Pauli’s principle is valid.
Therefore, we can finally write the electronic configuration of oxygen based on Hund’s rule and Pauli’s exclusion principle as 1s22s22p4.
Note: In the above electronic configuration the subshells 1s 2s 2p are arranged in increasing order of energy and is based on the Aufbau’s rule which states that electrons are added one by one into the various orbitals in order of their increasing energy starting with the orbital with lowest energy.
