Question
Question: How do you name halides?...
How do you name halides?
Solution
Hint : To answer this question firstly let us understand what we mean by halides. Halides are defined as the binary compounds in which one part is an element or group of elements and the other parts is the halogen atom. There are two types of halides possible. Halides can be organic halides as well as inorganic halides.
Complete Step By Step Answer:
To answer this question let us firstly see the naming of inorganic halides.
Inorganic halides are binary compounds which have one atom as halogen while another atom is basically any other element. The naming of such halides is very simple. Follow some basic rules.
Rule 1: For naming such halides we write the name of an element other than halogen in the compound as it is as a prefix and in the suffix, we add the name of the halogen atom. While writing the name of the halogen we need to convert (-ine to -ide). For example, chlorine is converted to chloride.
Few examples are as follows:
HCl is named as hydrogen chloride.
NaCl is named as sodium chloride.
Rule 2: If there are many halogen atoms present in the compound then we need to mention the oxidation state of the non-halogen element is brackets.
For example, FeCl is Iron (I) chloride whereas FeCl2 is Iron (II) chloride.
For organic compounds:
In organic chemistry, we have a class of halogen derivatives which are known as haloalkanes or alkyl halides. The naming of these organic compounds is based on the rules of the IUPAC. Let us see the rules for the nomenclature of alkyl halides.
Rule 1: Select the longest continuous chain containing the carbon attached to the halogen group and name it as the parent chain. If a double or triple bond is present, parent chain must contain it.
Rule 2: Number the carbon atom of the parent chain, beginning from the end nearer to the first substituent regardless of whether it is alkyl or halo group.
Rule 3: If two or more substituents are present then they are named in the alphabetical order along with their appropriate positions.
Rule 4: If two different substituents are present at the same position, from the two ends, then numbering of the chain is done in such a way that substituent which comes first in the alphabetical order gets a lower number.
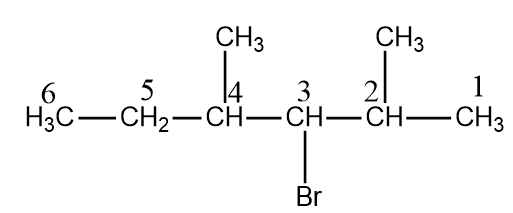
In this the parent chain is hexane as it is the largest chain. For numbering we will follow rule-2. Therefore, by rule-2 numbering starts from Right side of the chain. Apart from this there are other substituents methyl at (2 and 4) carbon atoms. Bromine at carbon – 3. Therefore, the name of the compound will be ( 3 -bromo- 2,4 dimethyl hexane).
Note :
It may be noted that in the above nomenclature since there were two methyl groups present therefore, we used the term ‘’di’’. Similarly, in IUPAC nomenclature ‘’tri’’ denotes three substituents. Also please note that while writing the name we have followed the alphabetical order.
