Question
Question: How do you name epoxides and ethers?...
How do you name epoxides and ethers?
Solution
In order to name epoxides and ethers firstly the classify the compounds, a compound in which two carbon atoms are connected to an oxygen atom likewise epoxides are the three membered, cyclic compounds. Also all ethers will also have a similar common name, which is often used as with the IUPAC name. If we want to acquire the common name, we have to name the two groups flanking the O atom in an alphabetized form and the word "ether" is added along with that.
Complete answer:
There are several ways of naming epoxides and ethers. In which epoxides is classified by two ways naming:
Substitutive names:These compounds we named as an alkane with an alkoxy alkane. Example (CH3)2CH−O−CH3 is 2−methoxypropane
Functional class names:R−O−R′ is named with the names of R and R′ in alphabetical order, followed by the class name "ether". Example (CH3)2CH−O−CH3 is ethyl isopropyl ether.
Also ethers is naming classified by-
Substitutive names:The compound is named as an alkene with an epoxy substituent. Example, 1,2−epoxybutane
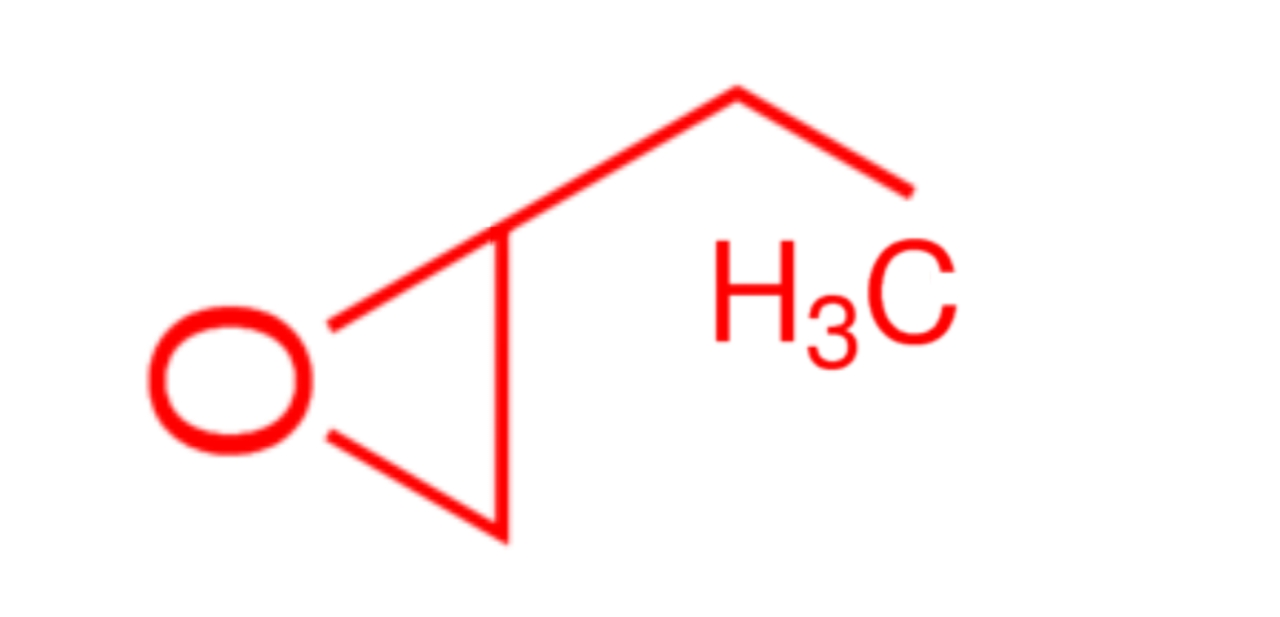
Functional class names:The compound is named as an epoxide of the corresponding alkene. Example, 1,2−epoxybutane is also named as but−1−ene epoxide
The Hantzsch Widman name:Here the ring we had named as a cyclopropane in which the O atom replaces a CH2 group indicated by the prefix "oxa" with the "a" which is being dropped when followed by a vowel. The three membered saturated ring is indicated by the prefix "ir" followed by the suffix "ane".
Therefore, 1,2−epoxybutane is called ethyloxirane. The O atom is automatically number 1 , so the ethyl group is on C−2 . We have no locating number is necessary here, because there is no such compound as 3−ethyloxirane
Oxide of the Correspondent alkene:Examples are ethylene oxide and propylene oxide. The first three methods are accepted by IUPAC.
The fourth is used only for simple alkenes such as ethylene, propylene, and butylene.
Note: Also note that thiols are compounds that are structurally identical to alcohols except that they replace the oxygen atom in the hydroxyl group with a sulfur atom. Likewise, sulfides are structurally identical to ethers, but they replace the oxygen atom with a sulfur atom, as shown below.
