Question
Question: How do you name and draw stereoisomers for \[2 - pentene\]?...
How do you name and draw stereoisomers for 2−pentene?
Solution
Stereoisomers have a similar primary equation yet the direction of the segment particles varies from one stereoisomer to the next. Two kinds of stereoisomers will be concentrated in this unit, mathematical isomers and optical isomers.
With regards to naming the stereoisomers the prefix cis or trans is embedded toward the beginning of the IUPAC name.
For instance 2−pentene can have two stereoisomers relying upon the direction of the alkyl group around the double bond.
cis−2−pentene and trans−2−pentene
Complete step by step answer:
The equation for finding the most extreme number of stereoisomers X will be X = 2n , where n is the quantity of stereogenic atoms in the particle. The formula X = 2n dependably gives the most extreme number of stereoisomers, however in circumstances of high symmetry it fails to give the real number.
cis−pent−2−ene has the H - particles on a similar side of the double bond. trans−Pent−2−ene has the H - molecules on inverse sides of the twofold bond. You can likewise utilize the Cahn-Ingold-Prelog framework to name the isomers. On each finish of the double bond, the alkyl gatherings (ethyl and methyl) have upper need than the H particles.
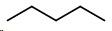
Pentane
Draw a double bond between carbons 2 and 3 .
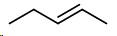
Pentene
You can also use the Cahn-Ingold-Prelog method to name the isomers.
On each end of the double bond, the alkyl groups (ethyl and methyl) have upper priority than the H atoms.
The first isomer has the higher-priority groups on reverse sides. This is (E)−pent−2−ene.
The second isomer has the higher-priority groups on the similar side. This is (Z)−pent−2−ene
Note: There are two ways to insert the double bond.
One has the H atoms on reverse sides of the double bond; the other has the H atoms on the similar side of the double bond.
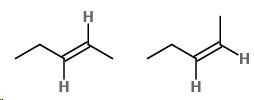
Cis/trans
cis−Pent−2−ene has the H-atoms on the similar side of the double bond.
Trans−Pent−2−ene has the H-atoms on reverse sides of the double bond.
The basic requirement for this stereoisomerism is that every carbon of the double bond should have two diverse substituent groups
