Question
Question: How do you name and draw 5 compounds that have the formula \( {C_7}{H_7}Br \) ?...
How do you name and draw 5 compounds that have the formula C7H7Br ?
Solution
To know the name of compounds which have the formula C7H7Br , we should first write the general formula of the given formula, then we will discuss the five compounds which have the given formula.
Complete answer:
Well, you know that an alkane would have the general formula CnH2n+2 , so this compound cannot be a straight-chained alkane.
That means you should try drawing alkenes and alkynes, and definitely try rings:-
∗ I tried a five-membered ring with two side-chain carbons, and added conjugated double bonds to decrease the number of hydrogens. That's one isomer.
∗ I tried drawing a six-membered ring with one side-chain carbon. When I placed a bromine on that carbon, I achieved C7H7Br . I moved the bromine onto the ring for another isomer, and moved that bromine around the ring for two more isomers for a total of five.
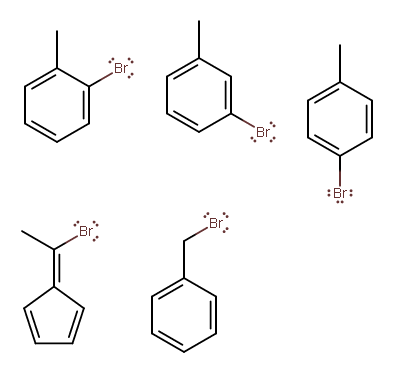
Seems like there are at least 14 though. If you want, three of them are bromene-ynes, which can be a challenge to name.
From left to right, top to bottom:
∗ 2−methyl−bromobenzene, oro−bromotoluene, or 1−bromo−2−methylbenzene
∗ 3−methyl−bromobenzene, orm−bromotoluene, or 1−bromo−3−methylbenzene
∗ 4−methyl−bromobenzene, orp−bromotoluene, or 1−bromo−4−methylbenzene
∗ I'm not sure how to name this one, so I looked it up and got:
\begin{array}{*{20}{l}}
{5 - \left( {1 - bromoethylidene} \right) - 1,3 - cyclopentadiene} \\\
\;
\end{array}
This uses cyclopentadiene as the parent compound, and an ethylidene substituent ( C=C∗−CH3 , with bromine on the starred carbon), and the upper-left carbon on cyclopentadiene is carbon-1; we'd move counter-clockwise.
Note:
Benzyl bromide is an organic compound with the formula C6H5CH2Br . The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties.
