Question
Question: How do you name alkenes using systematic names?...
How do you name alkenes using systematic names?
Solution
The molecular formula of a hydrocarbon provides information about the possible structural types it may represent
The alkenes are hydrocarbons which have carbon-carbon double bond functional groups and are unsaturated hydrocarbons with the molecular formula is CnH2nCnH2n, which is also the same molecular formula as cycloalkenes.
Alkenes are named as we do alkanes, but with the ending ene and giving the double bond the highest numbering priority.
Complete step by step answer:
The common family of hydrocarbons found in crude oil is the alkenes. In this family there is at least one carbon-carbon double bond. This double bond makes a big difference to the chemistry of the compounds of the family.
When naming alkanes:
Find the longest continuous chain of carbon that contains carbons of the double bond.
Give the lowest possible number to C=C or priority is given to carbon double bonds.
Add substituent and their positions to the name of the alkene as prefixes.
Identify stereoisomers.
For example
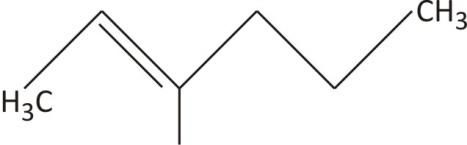
This a six carbon chain hexane with double bond between C2−C3. The base name becomes hex-2-ene.
The methyl group at C−5 changes the name to 5−methylhex−2− ene.
The full name for the compound is 5−methylhex−2− ene.
Note: The molecular formula of a hydrocarbon provides information about the possible structural types it may represent. For example, consider compounds having the formula C5H8C5H8. The formula of the five-carbon alkane pentane is C5H12C5H12 so the difference in hydrogen content is 4. This difference suggests such compounds may have a triple bond, two double bonds, a ring plus a double bond, or two rings. Hence a nomenclature system should be adopted. Alkenes can act as substitute groups. Alkanes with Unbranched carbon chains are simply named by the number of carbon in the chain
