Question
Question: How do you make acetic anhydride?...
How do you make acetic anhydride?
Solution
Acetic anhydride is an organic compound containing an anhydride functional group. The molecular formula of the acetic anhydride is C4H6O3.
It is a hazardous compound hence, not safe to use. It is highly corrosive, irritates skin and eyes.
At high concentration, it also damages the lungs and also affects the nose, throat, mounth. Hence, there is a need to handle it carefully.
Complete step-by-step answer:
Acetic anhydride possesses chemical formula is C4H6O3 and its structure is as follows:
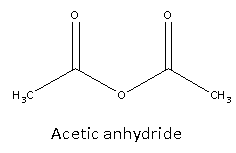
From structure, we can say that it is symmetric.
It is prepared from the acyl chloride and sodium salt of the acetic acid and the reaction is as follows:
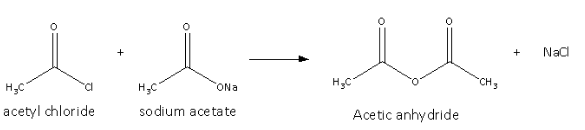
Here, we can see that the chlorine from the acetyl chloride and sodium from the sodium salt of acetic acid form sodium chloride.
Other methods are also used to prepare the acetic anhydride. Acetic anhydride is also prepared by the dehydration of the acetic acid at 800∘C. It also can be prepared by the carbonylation of methyl acetate.
Note: Based on functional groups present in the organic compounds different types are acids, alcohols, anhydrides, halides, ketones, aldehydes, etc.
Acetic anhydride belongs to the anhydride functional group.
Acetic anhydride is used as raw material for the synthesis of plastic and cellulose acetate fibers. It is also used as a raw material in pharmaceuticals for the synthesis of aspirin, acetaminophen, etc.
In chemical synthesis, it is widely used as an acetylating agent which causes acetylation of the material.
It affects the different parts of the body like skin, lungs, mouth, nose, throat, etc hence, there is a need to handle it carefully.
