Question
Question: How do you identify the compound for IR Spectrum for the molecular formula \[{C_5}{H_8}O\]?...
How do you identify the compound for IR Spectrum for the molecular formula C5H8O?
Solution
We have to know that the IR range starts from 800 to 4000cm−1. We can observe and measure this “singing” of bonds by applying IR radiation to a sample and measuring the frequencies at which the radiation is absorbed. The result is a technique known as Infrared Spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule.
Complete answer:
IR-frequency light is passed through a compound. The amount and frequencies of the light absorbed is related to the functional groups and structure of the compound. This helps us to identify the compound. All “spectroscopy” methods use light wavelengths from infrared to UV.
The compound with molecular formula C5H8O, contains oxygen atoms. The probable compound must have a ketonic group in it as it has one oxygen atom. Also we can look for aldehydes and alcohols as they also contain one oxygen atom.
So when we derive the structure for this compound we get: This can be the most probable compound having molecular formula C5H8O.
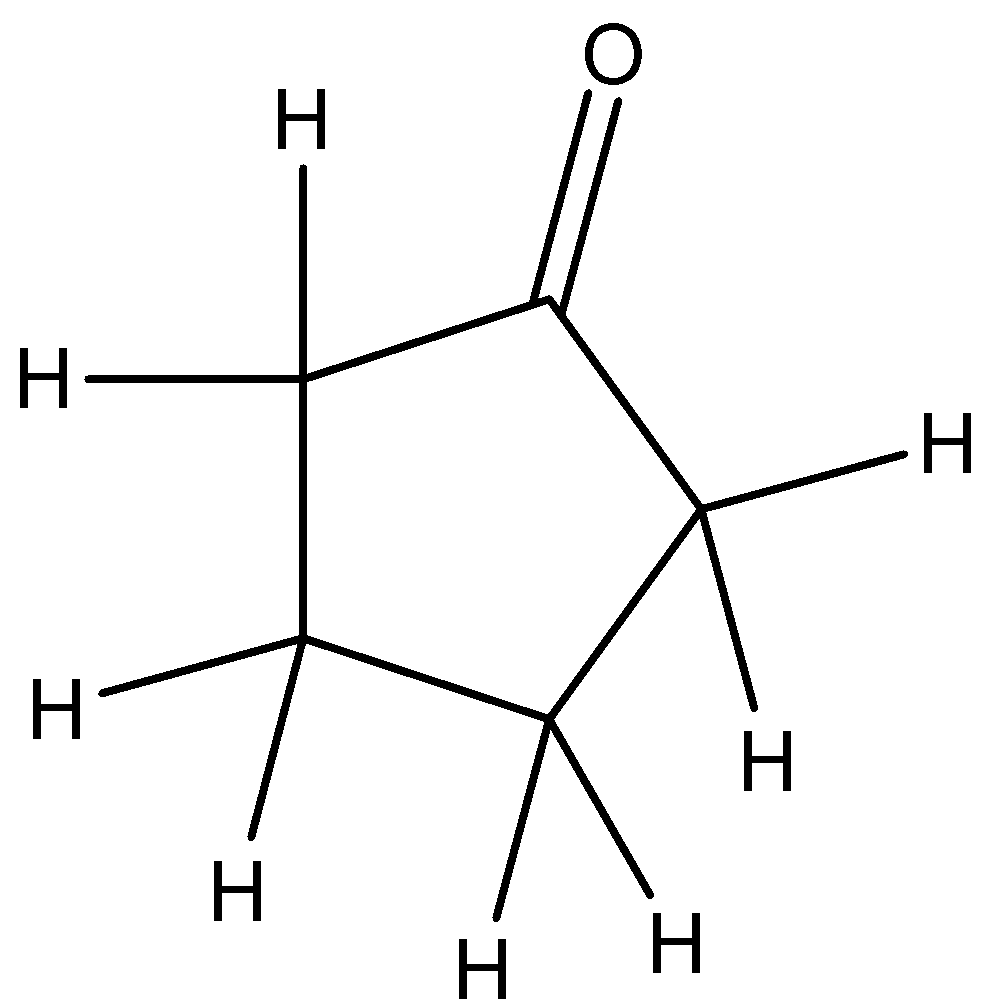
The Infra-Red (IR) spectrum of acyclic ketones shows a strong C=O stretching absorption at 1715cm−1.
The other important vibrational peak of cyclopentanone molecule is of carbon-hydrogen bond, which is at 3000cm−1. At 1500cm−1, saturated carbon- carbon bending mode vibrations occur. In the fingerprint region, carbon hydrogen wagging mode vibrations occur.
Note:
As we move from acyclic to cyclic ketones the value of this absorption rises as the ring-size decreases from 6 to 3 .Cyclic ketones don't terminate with methyl groups but only show signals and splitting pattern for methylene groups which make up their ring structure.
