Question
Question: How do you draw the structural formula of ethanol?...
How do you draw the structural formula of ethanol?
Solution
Ethanol is a compound of carbon, hydrogen, and oxygen components were portrayed by Antoine Lavoisier and its structural formula was controlled by Nicolas-Theodore de Saussure in 1808. After five years, Archibald Scott Couper distributed the primary equation of ethanol. A blend of 95% ethanol and 5% water is the ethyl alcohol utilized in powers and virtually all mechanical exercises. Incredibly harmful are both outright and 95 percent ethanol.
Complete step-by-step answer:
Ethyl alcohol is a colorless, volatile fluid with a trademark scent and an impactful taste. It has a flashpoint of55∘F, is named a depressant medication, and is harmful when ingested in enormous amounts.
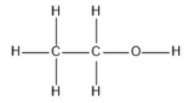
The sub-atomic formula portrays the creation of ethanol particles, two carbon atoms, six hydrogen atoms and one molecule of oxygen that happens to each particle yet gives us no underlying data. Underlying data is the manner by which the particles are associated and how the atom occupies the space.
The carbon atoms in ethanol are sp3 hybridized for example they have a free rotation. It is the second simplest alcohol after methanol and represented as:
Note:
i) Ethanol is utilized in mixed drinks, solvents, fragrances, flavorings, shading, medications, substance combination, and thermometers.
ii) Ethanol is utilized as a fuel, a magnificent dissolvable, and significant crude material for making different synthetic compounds.
iii) Unadulterated ethanol is a poisonous fluid, methanol which is considerably more harmful is sometimes added to it to put off anybody needing to drink it.
