Question
Question: How do you draw the Lewis structure of the molecule containing one C and four F atoms?...
How do you draw the Lewis structure of the molecule containing one C and four F atoms?
Solution
Lewis dot structure also called the electron dot structure. They represent the valence electron of the atoms present within the molecule. By Lewis dot structure we can visualize the bonding electrons and the lone pair of electrons present in the molecule.
Complete step by step answer:
In the question, the compound that is given contains a single carbon atom and four fluorine atoms. Since carbon is a tetravalent atom thus we get the compound as carbon tetrafluoride.
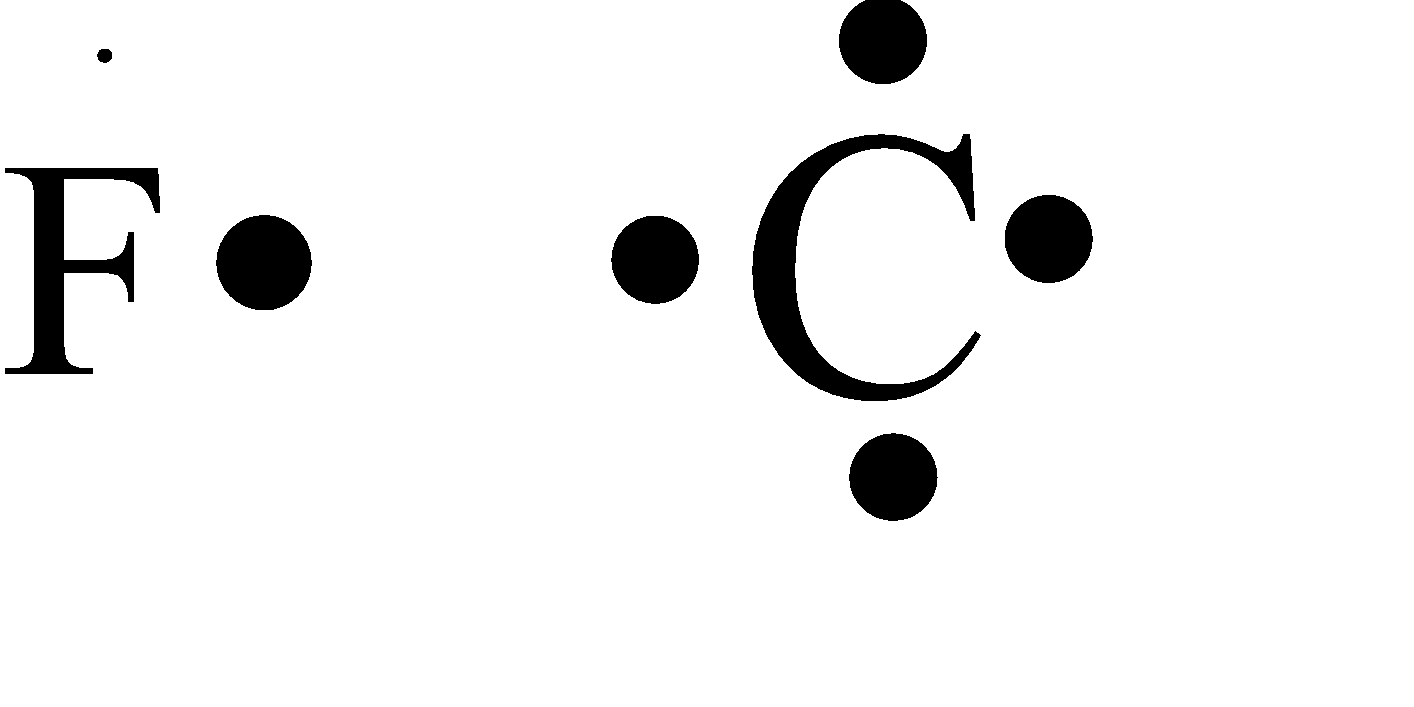
Carbon is tetravalent this will contain four electrons in the valence electron shell. While the fluorine is of group 17 belonging to the halogen family and thus will form the valence electron of one.
All the valence electrons of the four fluorine present which are single will bond with among the four electrons of the carbon and thus will form a single bond with the carbon atom.
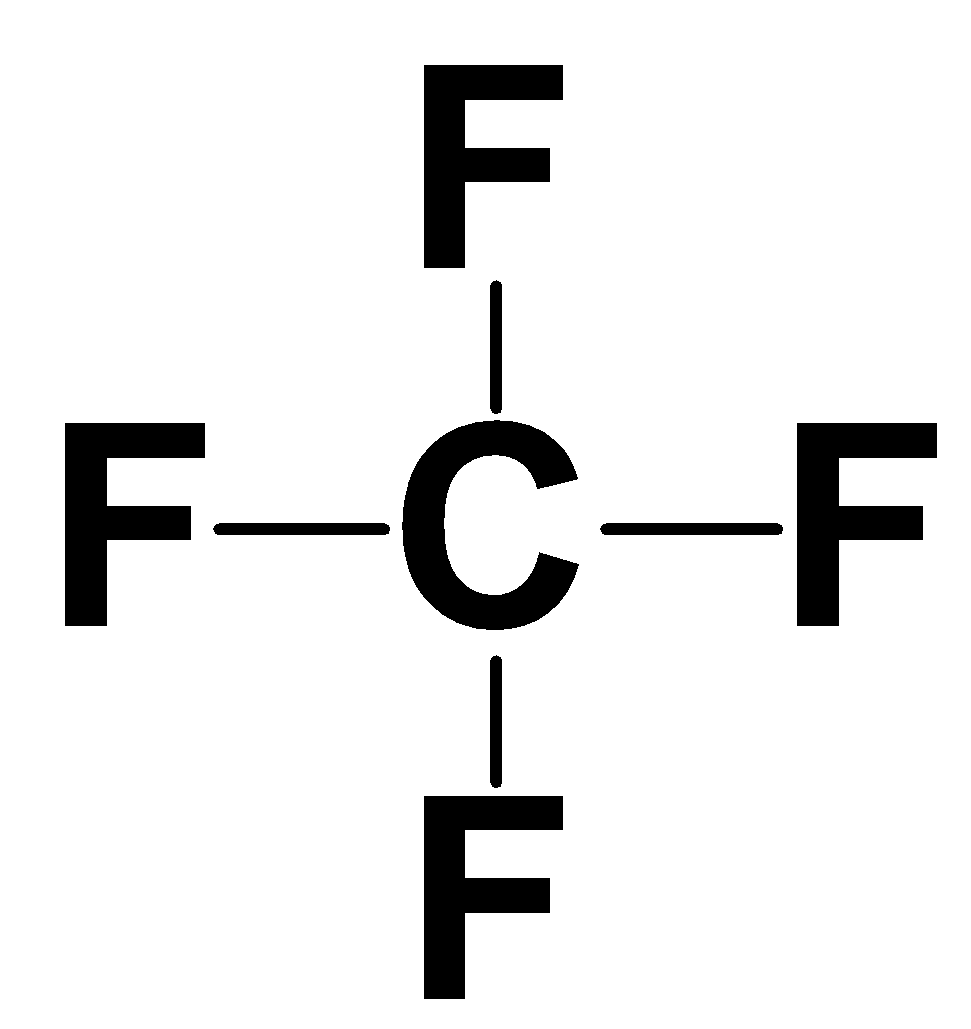
Note: Carbon is a tetravalent atom which means it has four valence electrons and thus can form four covalent bonds with atoms. Based on this property the carbon atom can be categorised as primary, secondary or tertiary.
The carbon which is attached to only one other carbon atom is called primary carbon, the carbon atom with the attachment of two other carbon atoms is secondary carbon whereas the carbon atom with the attachment of three carbon atoms is termed as a tertiary carbon atom.
By making the Lewis dot structure of the molecule we may determine the formation of the chemical bonds in various molecules. We may draw the Lewis structures for the molecules containing the covalent bonds and also can draw them for the coordination structures.
