Question
Question: How do you draw the Lewis structure of \({\text{P}}\)?...
How do you draw the Lewis structure of P?
Solution
Hint For writing the Lewis structure of the Phosphorous, we have to know about the electrons which are present in the outermost shell of this atom and formal charge on this phosphorus atom if it is present in the molecule.
Complete step by step solution:
Some points which we have to know before constructing the Lewis structure of Pare as follow:
-In the periodic table phosphorus is denoted as 1530.9P and situated at ‘V A’ group in the p – block of the table.
-From the denotation it is clear that atomic number of Phosphorus (P) atom is 15 and we know that atomic number of an atom is always equal to the number of protons or number of electrons.
-So, in the phosphorus atom 15 electrons are present and electronic configuration is written as [Ne]3s23p3 and from this electronic configuration it is clear that in the outermost shell of Phosphorus five valence electrons are present.
-Now we calculate formal charge on phosphorus atom by the formula as given below:
Formal Charge = Total no. of valence electrons – Non bonded electrons – No. of bonds
-Formal charge on phosphorus atom = 5−5−0=0
So, the Lewis dot structure of phosphorus atom is shown as follow by keeping all above points in mind:
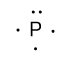
Note: Here some of you may think that when in phosphorus atoms five valence electrons are present in the outermost shell then why only one lone pair is showing, why two lone pairs are not showing. So the reason is that in p-orbital three electrons are present in unpaired form not in paired form.
