Question
Question: How do you draw the Lewis structure for the molecule \(HBr\)?...
How do you draw the Lewis structure for the molecule HBr?
Solution
For writing the Lewis structure of the hydroxide ion, we have to know about the electrons which are present in the outermost shell of each atom involves in the molecule and the formal charge of each atom present in the molecule.
Complete answer:
Some points which we have to know before constructing the Lewis structure of HBr are as follow:
-Atomic number of bromine (Br) atom is 35 and its electronic configuration is written as [Ar]4s23d104p5 and from this electronic configuration it is clear that in the outermost shell of bromine seven valence electrons are present.
-Atomic number of hydrogen (H) atom is one and its electronic configuration is written as 1s1 and from this electronic configuration it is clear that in the outermost shell of hydrogen one valence electron is present.
-In HBr single bond is present between hydrogen atom and bromine atom.
Now we calculate formal charge on each atom of molecule by the formula as given below:
Formal Charge = Total no. of valence electrons – Non bonded electrons – No. of bonds
-Formal charge on Hydrogen atom = 1−0−1=0
-Formal charge on Bromine atom = 7−6−1=0
So, the Lewis dot structure of HBris shown as follow by keeping all above points in mind:
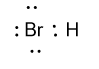
Note:
Here some of you may do wrong during calculating the formal charge by considering lone pairs of electrons as one electron. But always keep in mind we have to count each non bonded electron separately, not in pairs.
