Question
Question: How do you draw the Lewis structure for \(P{{H}_{3}}\) ?...
How do you draw the Lewis structure for PH3 ?
Solution
Lewis dot structures also known as electron dot structures. Lewis dot structures represent the valence electrons of the atoms present within the molecule. By Lewis dot structures we can visualize the bonding electrons and lone pair of electrons present in the molecule.
Complete answer:
- In the question it is given that to draw the Lewis dot structure of the phosphine.
- We know that the atomic number of phosphorus is 15.
- The electronic configuration of phosphorus is 1s22s22p63s23p3 .
- So the phosphorus atom has three valence electrons in 3p orbital.
- The Lewis dot structure of the phosphorus is as follows.
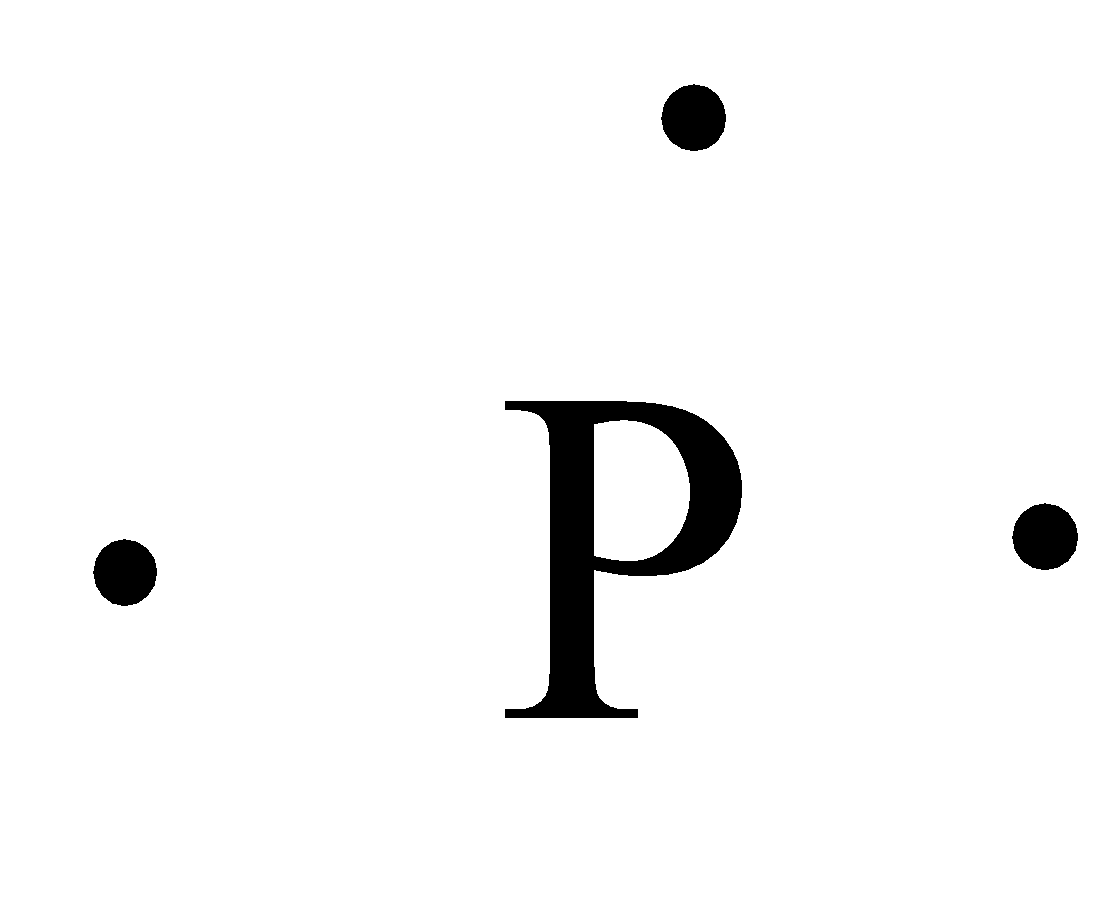
- Coming to hydrogen, we know that the hydrogen has one valence electron in its electronic configuration.
- The Lewis dot structure of hydrogen is as follows.
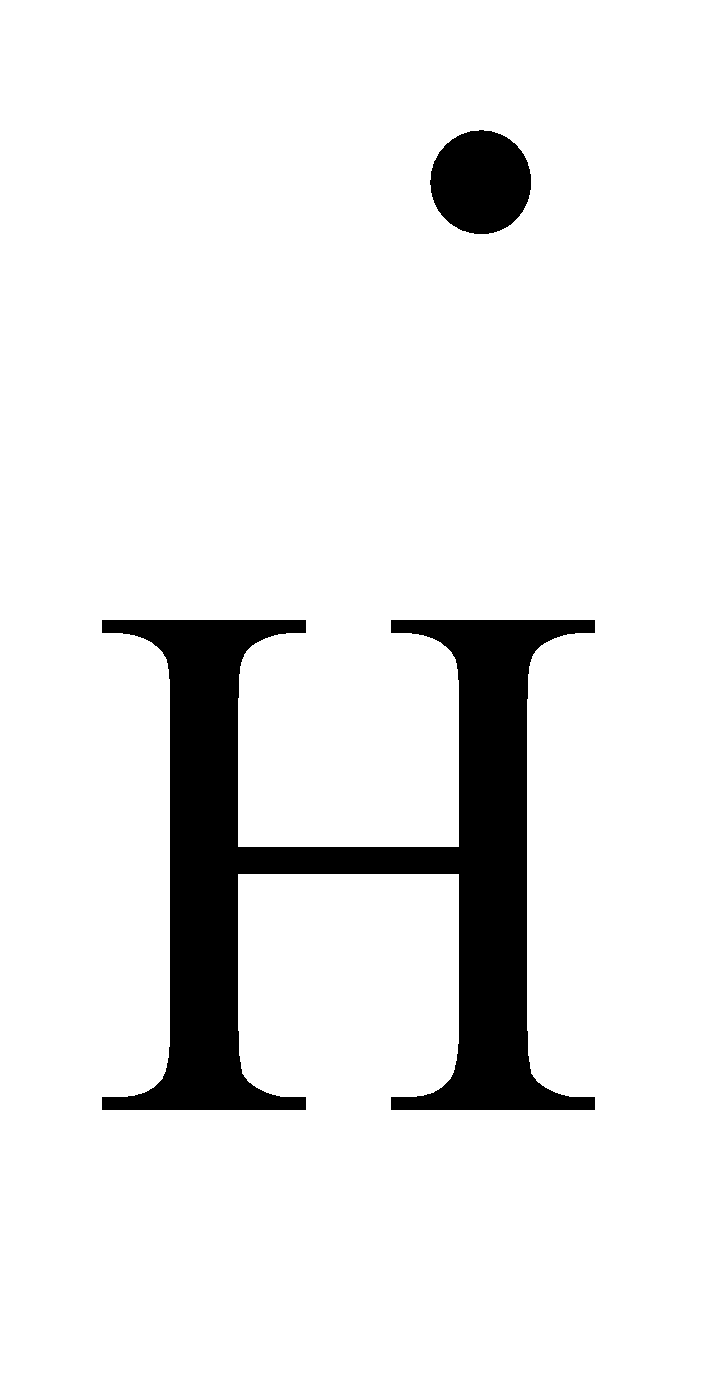
- Now by taking the above structures as examples we can draw the Lewis dot structure of PH3 and it is as follows.
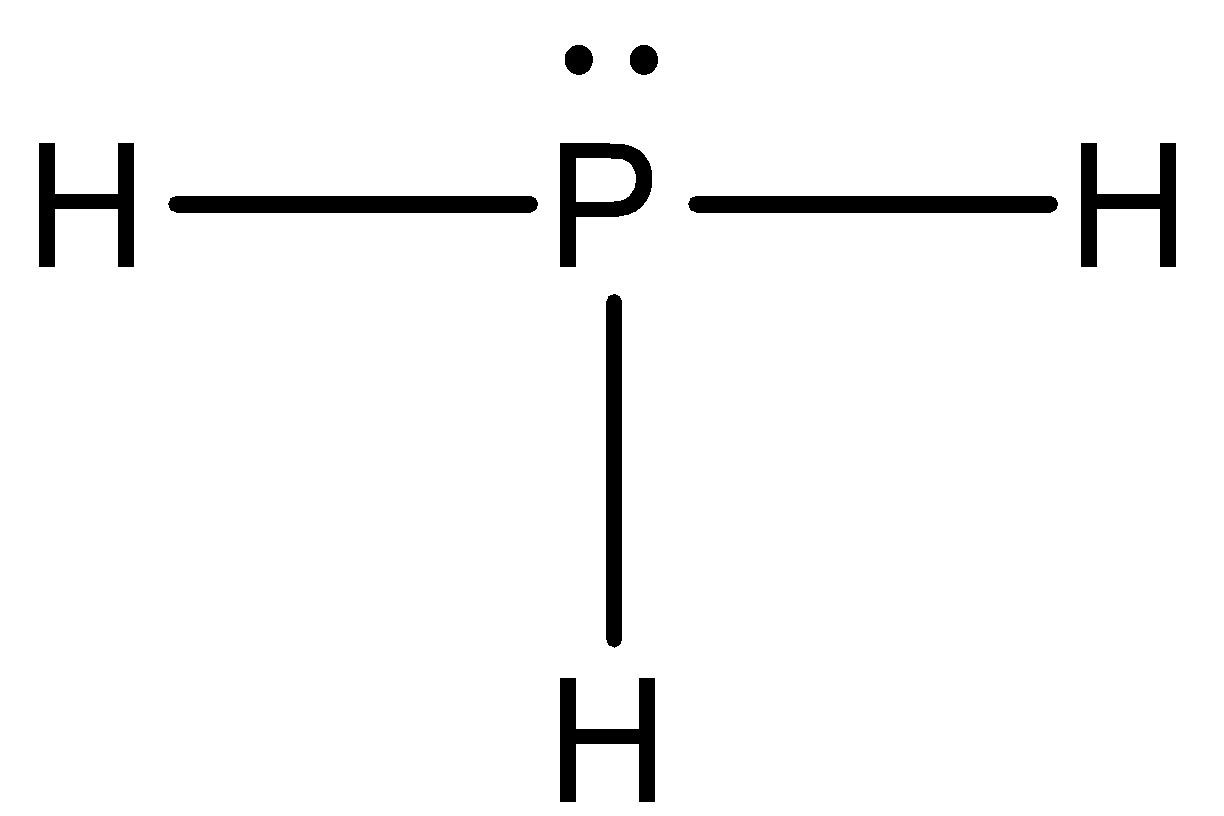
- From the above Lewis dot structure we can easily say that there are three covalent bonds present in phosphine molecules and one lone pair of electrons are present on phosphorus which are because of 3s electrons in the electronic configuration of phosphorus atoms.
Note:
By using Lewis dot structures of the molecules we can determine the formation of the chemical bonds in various molecules. We can draw the Lewis dot structures for the compounds containing covalent bonds and also we can draw for coordination compounds.
