Question
Question: How do you draw the Lewis dot structure for \(Ca{I_2}\) ?...
How do you draw the Lewis dot structure for CaI2 ?
Solution
We have to remember that a Lewis Structure is an extremely improved portrayal of the valence shell electrons in a particle. Lewis dot structure is utilized to show how the electrons are masterminded around singular particles in an atom. Electrons are displayed as "specks" or for holding electrons as a line between the two particles. The objective is to get the "best" electron design, for example the octet rule and formal charges should be fulfilled.
Complete answer:
Lewis structures show every particle and its situation in the design of the atom utilizing its compound image. Lines are drawn between molecules that are attached to each other (sets of specks can be utilized rather than lines). Abundance electrons that structure solitary sets are addressed as sets of specks, and are set close to the molecules.
Albeit fundamental gathering components of the subsequent period and past ordinarily respond by acquiring, losing, or sharing electrons until they have accomplished a valence shell electron setup with a full octet of electrons, hydrogen can just shape securities that share only two electrons.
The Lewis structure of calcium iodide is,
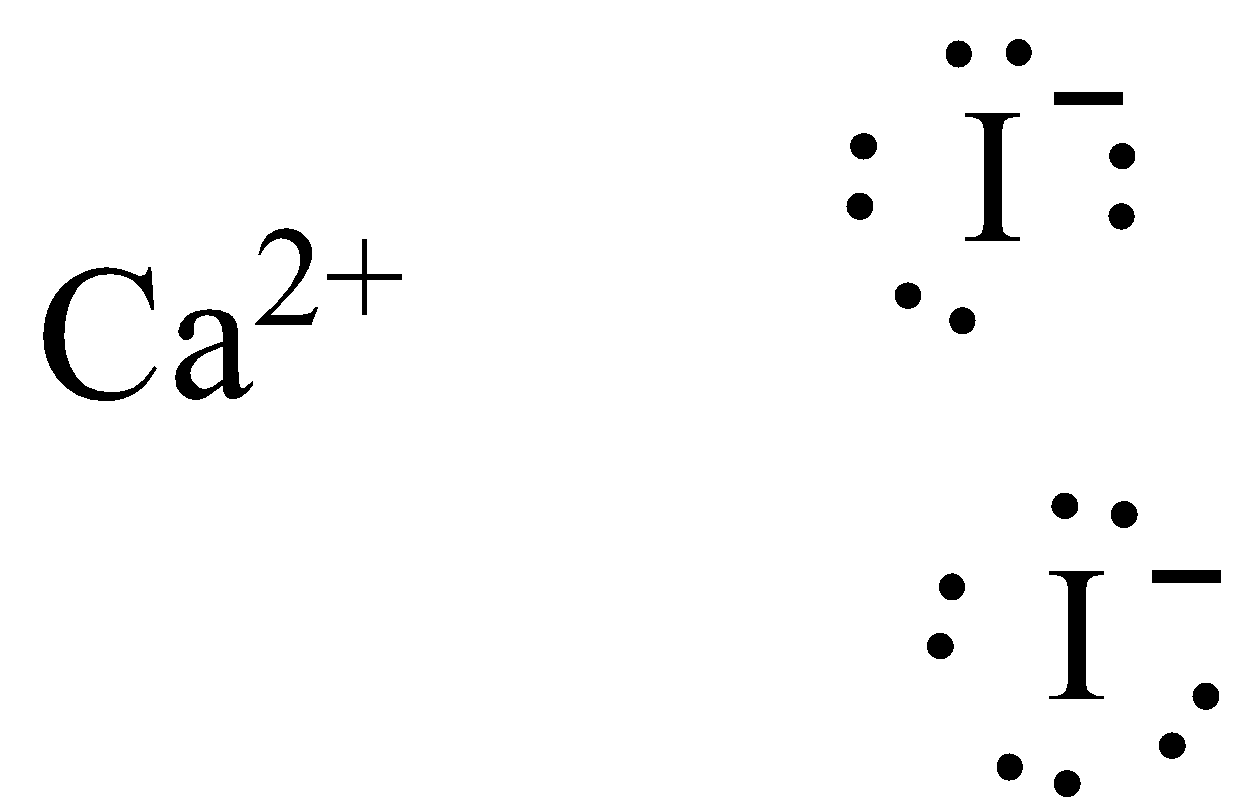
Note:
We need to know that the calcium iodide (synthetic equation CaI2) is the ionic compound of calcium and iodine. This vapid deliquescent strong is a salt that is exceptionally solvent in water. Its properties are like those for related salts, like calcium chloride. It is utilized in photography. It is likewise utilized in feline food as a wellspring of iodine. By treating calcium carbonate, calcium oxide, or calcium hydroxide with hydroiodic acid calcium iodide can be produced. Calcium iodide gradually responds with oxygen and carbon dioxide noticeable all around, freeing iodine, which is liable for the weak yellow shade of unclean examples.
