Question
Question: How do you draw the cis and trans isomers for 1-bromo-4-chlorocyclohexane?...
How do you draw the cis and trans isomers for 1-bromo-4-chlorocyclohexane?
Solution
Isomers are compounds with the same molecular formula but the different structural formula and different properties/arrangements in space. Isomerism is the process of holding isomers and it can be classified into different types as structural, functional, stereo, and geometrical isomerism based on its structure, arrangement, and properties. The well-known example of isomers is ether and alcohol which are functional isomers with each other. Cis and Trans are the isomers based on the position of which substituents get added.
Complete step by step answer:
cis isomers are the compound in which the substituents are facing towards the same side i.e. substituents are present either front side or backside of the atom attached. The cis structure for 1-bromo-4-chlorocyclohexane can be drawn as,
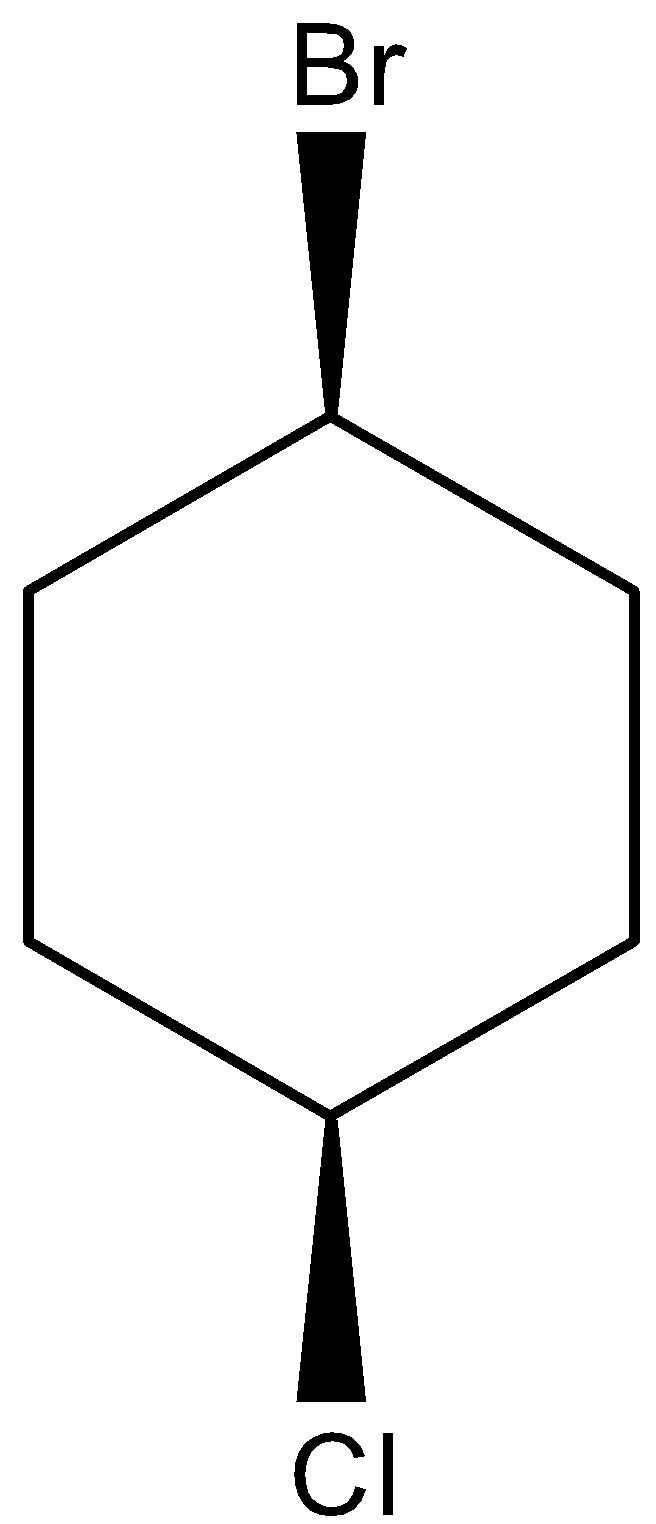
Similarly, trans isomers are the compound in which the substituents are facing opposite to each other. i.e. if one substituent is present at the front side of the atom attached, then the other substituent will be present on the backside of the atom attached. The trans structure for 1-bromo-4-chlorocyclohexane can be drawn as,
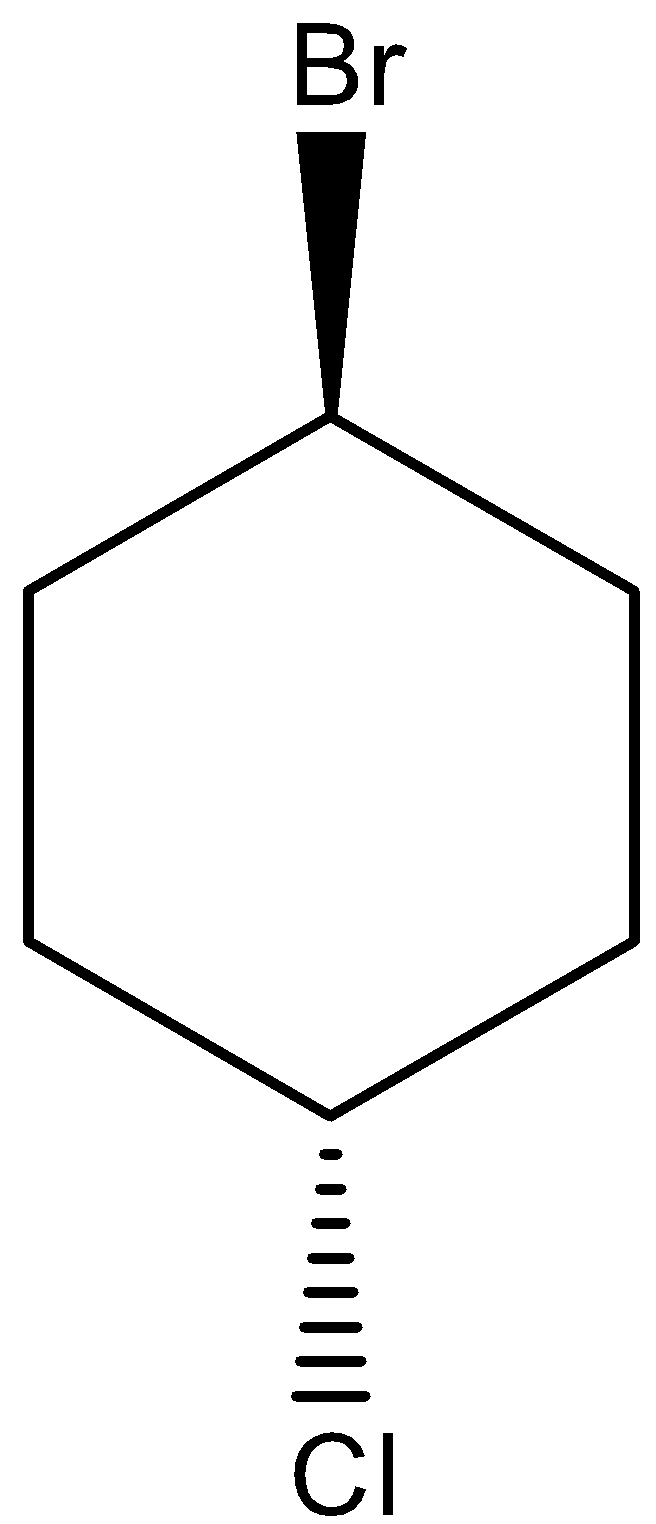
Note: Structural isomers are the compound with the same molecular formula but different structural formula. Under structural isomerism, functional, chain, and tautomer are present. Stereoisomers are the compound with the same molecular formula but different orientation in space. Under stereoisomers, geometry and optical isomers are present. Usually, in acyclic compounds, trans isomers are most stable than cis isomers due to the absence of steric effect in Trans isomers. But, in cyclic compounds, cis cycloalkanes are more stable than trans cycloalkanes since trans cycloalkanes show high ring strain than cis cycloalkanes.
