Question
Question: How do you draw sigma and pi bonds?...
How do you draw sigma and pi bonds?
Solution
Hint : A chemical bond formed by mutual sharing of electron pairs between the atoms is known as a covalent bond. These electron pairs are referred to as bonding pairs or a shared pair of electrons. The covalent bonds are directional i.e., atoms bonded via covalent bond are present in specific orientation with respect to one another.
Complete Step By Step Answer:
A covalent bond is broadly categorized into two types: sigma bond and pi bond. Let us discuss each type of covalent bond separately.
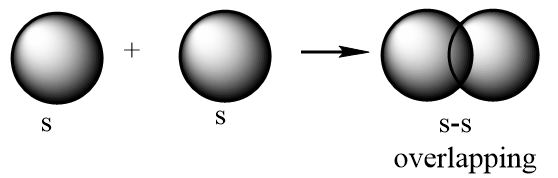
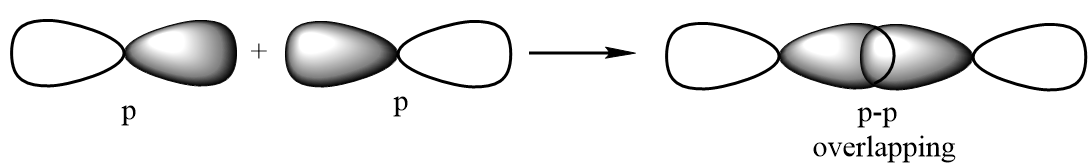
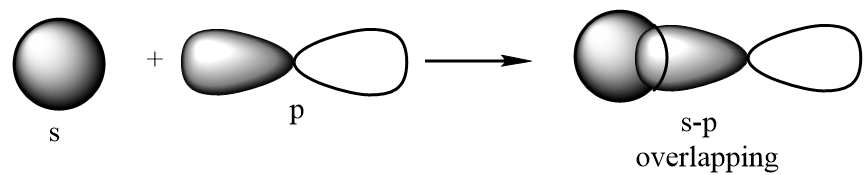
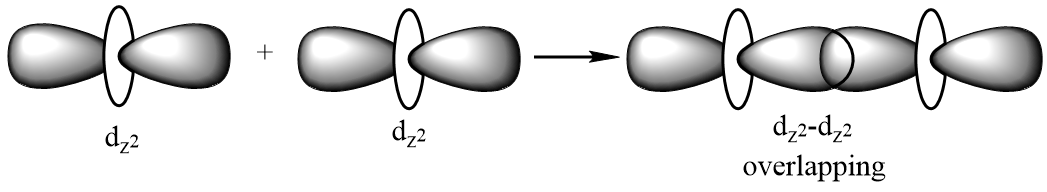
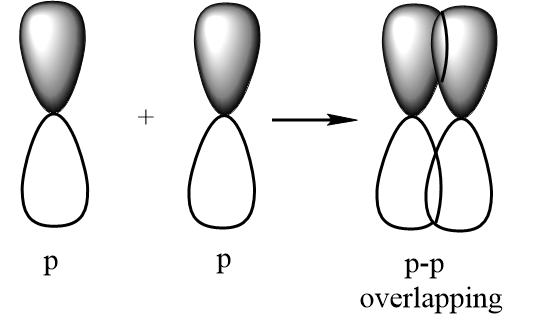
Sigma bond: A covalent bond which is formed as a result of formation of a molecular orbital by positive overlapping of atomic orbitals done by head-on-head or end-to-end overlapping. It is represented by a symbol σ. Possible illustrations for formation of a sigma bond are given below:
s-s overlapping:
p-p overlapping:
s-p overlapping:
dz2−dz2 overlapping:
Pi bond: A covalent bond which is formed as a result of formation of a molecular orbital by positive overlapping of atomic orbitals done by side-to-side overlapping. It is represented by a symbol π. Possible illustrations for formation of a pi bond are given below:
p-p overlapping:
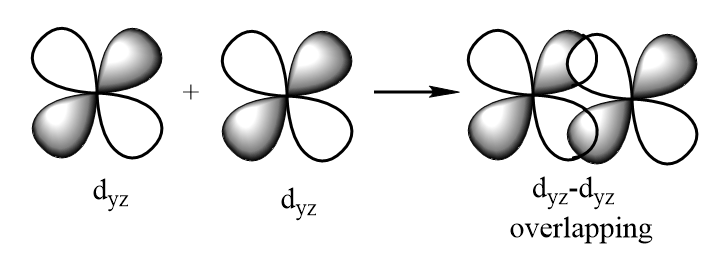
dyz−dyz overlapping:
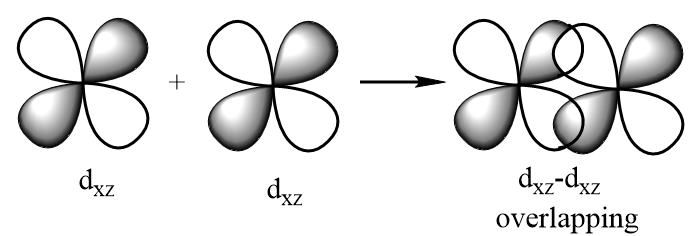
dxz−dxz overlapping:
Note :
It is a general misconception that sigma bonds are formed by overlapping of s-orbitals only and the pi-bonds are formed by the overlapping of only p-orbitals but the fact is a sigma bond can be formed by head-on-head overlapping of any orbital, not just s-orbital. Also, remember that a single bond in covalent bonding always consists of only sigma bonds whereas in double and triple bonds, pi bonds are also present along with a sigma bond.
