Question
Question: How do you draw propyne?...
How do you draw propyne?
Solution
You have three carbons associated, and two of them are fortified through one triple bond. Fill in the 4 hydrogens by knowing the valency of carbon as 4. The atomic equation of propyne, C3H4 , determines 2 levels of unsaturation; two double bonds as required.
H3C−C≡CH
Complete step by step answer:
Propyne is the second least difficult individual from the acetylene family. It can shape explosives combinations with air and oxidizing specialists. It is utilized as welding light fuel. Propyne shows up as a dreary melted gas with a sweet scent. mp: − 104∘C, bp: − 23.1∘C. Insoluble in water, dissolvable in ethanol, chloroform and benzene.
Taking a gander at the prefix, signifies three, so you have three carbons.
Taking a gander at the addition, signifies it's an alkyne, so you have in any event one triple bond. The overall equation for a straight-bonded alkyne is CnH2n−2 , so, you ought to get C3H4.
There isn't anything else in the name, so there is just one triple bond.
Accordingly, you have three carbons associated, and two of them are bonded by means of one triple bond. Fill in the 4 hydrogens by knowing the valency of carbon as 4
This would be propyne.
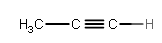
Had you drawn: H2C=C=CH2
That would be allene, which is mistaken. There are not two double bonds. It's an alkyne, not an alkene.
Propyne can likewise be combined on a research facility scale by lessening propanol, allyl liquor or (CH3)2CO fumes over magnesium. It is named utilizing a similar stem as the alkane having similar number of carbon particles however finishes in − ene to distinguish it as an alkene. In this manner the compound CH2=CHCH3 is propyne.
Note:
The first is a ring called cyclopropane. It is additionally conceivable to attract three carbons in a line with a double bond. Both Lewis structures are right for C3H6. In the wake of deciding the number of valence electrons there are in C3H6, place them around the focal atom to finish the octets.
