Question
Question: How do you draw ethyl methyl amine?...
How do you draw ethyl methyl amine?
Solution
If we know the IUPAC nomenclature of the hydrocarbons thoroughly then we could easily decode the structure from the name given. The functional group present is amine group which is −NH2.
Complete step-by-step answer: From the time we study organic chemistry, we study various types of hydrocarbons and its reactions, structure etc.
Here in the question we are supposed to draw the structure of methyl ethyl amine.
For drawing a structure for a molecule, from its name either it should the name and structure of lattice thoroughly or should have the idea on IUPAC nomenclature and try to decode the structural units involved in the reaction.
The method of decoding the structure from the given word is as follows. First we should identify the parent carbon chain which is the longest one and we know that with the number of carbon atoms varies, the prefix changes. And then the substituents given should be identified and the position of the carbon to which the substituent is attached too will also be mentioned in the name of the compound. And in some molecules functional groups are also present and in some multiple bonds too is present.
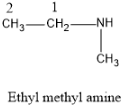
Now let us talk about the given molecule the name given is ethyl methylamine. The IUPAC nomenclature of the molecule is N-methyl-1- ethanamine.
So the parent carbon skeleton is of ethane i.e. which possesses two C atoms and it is attached to the functional group amine. Then the methyl group which is the substituent specified in the IUPAC naming is attached to the N atom of the amine group.
And the structure of the molecule will be as:
Note: Ethyl methyl amine is a secondary amine, since the amine group is attached to two alkyl chains. And the amine are basic in nature. We could distinguish between the primary, secondary and tertiary amines by carrying out reaction with nitrous acid.
