Question
Question: How do you draw enantiomers with a perspective formula?...
How do you draw enantiomers with a perspective formula?
Solution
Enantiomers are referred to as chiral molecules which are optically active. A pair of enantiomers are mirror images of one another which are non-super imposable.
Complete step by step answer: Perspective formula is a three dimensional representation of a chiral molecule. This represents a geometric representation of the molecule as a model structure which resembles the bonds in appropriate directions as the molecules really are.
Perspective formula is used primarily for drawing enantiomers in a piece of paper. Actually the three dimensional structure is drawn in two dimensions. The perspective formula of a molecule contains four different bonds around the asymmetric centre atom.
Out of the four bonds two bonds are placed on a plane. The third bond is drawn as a solid wedge and the fourth bond is drawn as a dashed wedge. The solid bond indicates that the bonded atom/group is placed above the plane of the paper and the dashed bond indicates that bonded atom/group is placed behind the paper.
As for example let us consider a chiral molecule as 2-bromobutane. The molecule is chiral as four different groups are attached to a chiral centre. The groups are CH3, −Br, −H and CH2CH3. The perspective formula of the pair of stereoisomers is as follows:
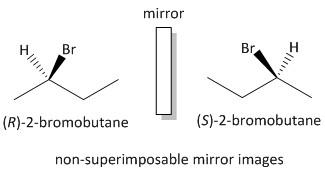
The two perspective formulas of the 2-bromobutane are non-superimposable mirror images of one another. For drawing the two enantiomers we often interchange the positions of two groups. Also we often change the two groups which are bonded by solid and dashed wedges.
Note:
Enantiomers are not separated by physical separation techniques as they have similar melting point, boiling point, infrared absorptions and NMR spectra. When two enantiomers are present in equal amounts the mixture is termed as a racemic mixture.
