Question
Question: How do you draw electron orbital diagrams?...
How do you draw electron orbital diagrams?
Solution
The atom contains orbitals in its structure and the electrons are going to revolve around the nucleus in fixed orbits called stationary orbits around the nucleus. Electrons have two spin clockwise and anti-clockwise.
Complete step by step answer:
- In the question it is asked how we can draw the electron orbital diagram.
- There are lots of ways to represent the electron orbital diagram.
- One of the ways to represent the electron orbital diagram is as follows.
- Here we are going to use lines to represent orbitals and arrows to represent electrons.
- Take an example of Helium, it contains 2 electrons in 1s orbital.
- The representation of the electron orbital diagram for helium is as follows.
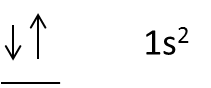
- The electrons in 1s orbital in opposite directions, one electron represented in clockwise direction and another electron represented in anti-clockwise direction.
- If we take a carbon element there are 6 electrons, the representation of electron orbital diagram for carbon atom is as follows.
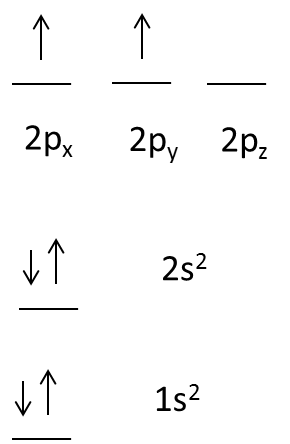
- In carbon first two electrons are present in 1s orbital and next two electrons are going to enter 2s orbital.
- After filling the electrons in 2s orbital the remaining electrons are going to enter into 2p orbital which contains three subshells.
- This is how we can represent the electron orbital diagrams for different atoms.
Note: At the time of writing electron orbital diagrams we have to follow the electronic configuration of the particular atom. We should know the number of electrons in a particular atom to draw the electron orbital diagram.
