Question
Question: How do you draw and label a Bohr model for \(N\)?...
How do you draw and label a Bohr model for N?
Solution
The answer here is based on the calculation of number of protons, neutrons and the electrons and then drawing the Bohr’s model accordingly whether the nucleus has protons and neutrons accommodated in it.
Complete step by step answer:
In the classes of chemistry, we have come across the concepts of atoms and also some the definitions relating to it such as the atomic number, atomic mass, composition of atom, properties of the atom etc.
Let us now focus on the Bohr’s atomic model and how it is represented.
- Bohr’s atomic model consists of the central nucleus which is drawn as the circle and then the total number of protons and neutrons are written accordingly.
Next, the first outer shell is drawn in which according to the electronic configuration rule, only two electrons can be accommodated.
- After the first shell, the next shell is drawn and this is a continuous process where it is different for every different atom.
Now, in the above given atom that is nitrogen N,
The atomic number of this element is 7. This means that there are a total of 7 protons in the nitrogen atom because the atomic number is nothing but the total number of protons in it.
Now, since the number of protons and electrons are always equal in any atom, the total electrons in nitrogen is 7.
- Number of neutrons can be calculated using the formula, Number of neutrons = mass number – number of protons. Now since the mass number of nitrogen is 14, the total neutrons is 14−7=7
Therefore, based on all these facts above, we can draw the Bohr’s atomic model for nitrogen as shown below:
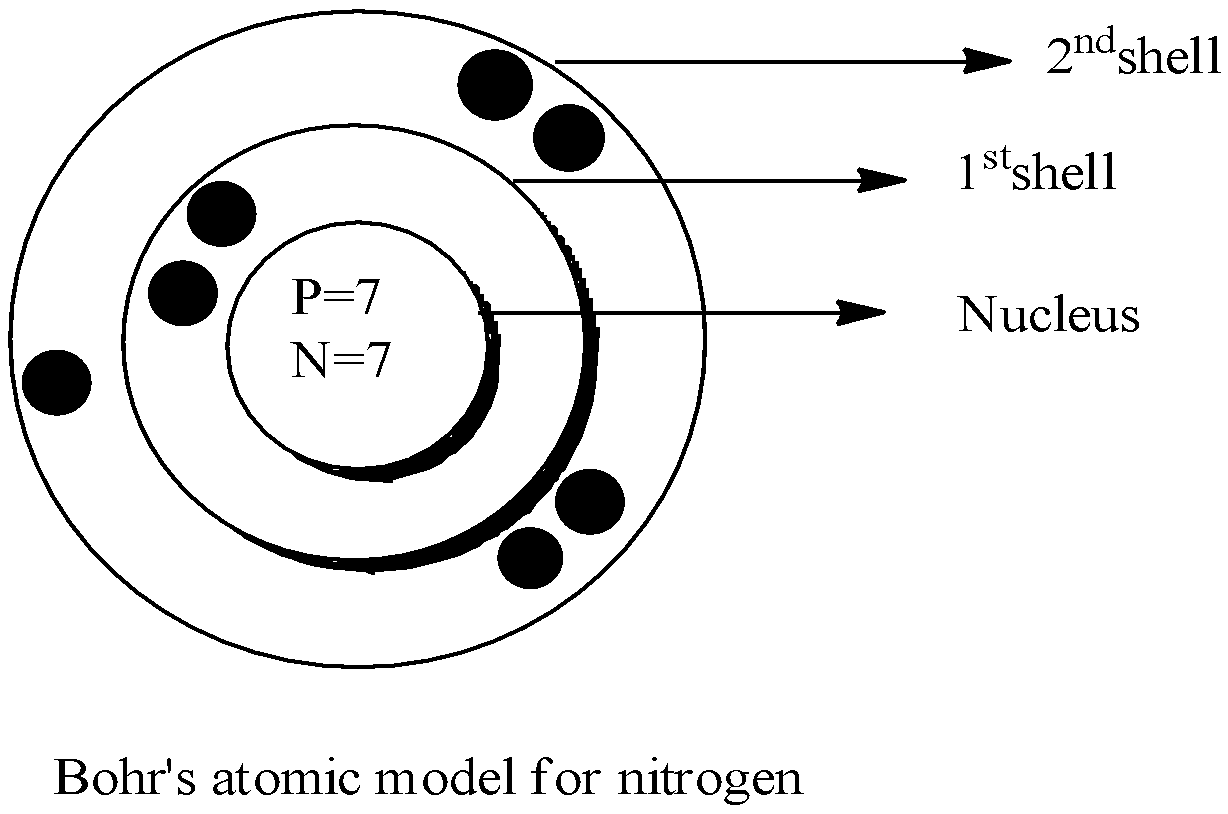
Note: Note that the shells denoted as first, second are nothing but the main shells that are K, L , M and N and not the sub shells that are s, p, d and f and do not confuse this term.
