Question
Question: How do you draw a Newman projection of the most stable conformation 2R-3S-dibromobutane sighting dow...
How do you draw a Newman projection of the most stable conformation 2R-3S-dibromobutane sighting down C2−C3 bond? Is this optically active, racemic, dextrorotatory or a meso compound?
Solution
The answer is based on the stereochemistry part of the organic chemistry section which says that Newman projections are written with the front carbon as dot and the rear carbon as a circle.
Complete step by step answer:
- In the lower classes of organic chemistry, we have studied about the types of writing the structure of the molecule in the 3D form which comes under stereochemistry such as Newman projection, Fischer projection, Sawhorse projection and so on.
- We shall now see in detail about the Newmann projection and how can 2R-3S-dibromobutane sighting down C2−C3 bond be written.
- Stereochemistry is the study of a molecule in space in the three dimensional form and hence this is also called as 3D chemistry.
- Among the different types of projections, the Newmann projection is written in a way where the front carbon is represented as a dot and back carbon as a circle and the cyclic molecules are written in the form of their conformations.
- Optically active compounds are those which can rotate the plane of the polarized light and here in this case even if both the carbon 2 and 3 are chiral, the optical activities cancel each other and there is no net optical activity in this compound.
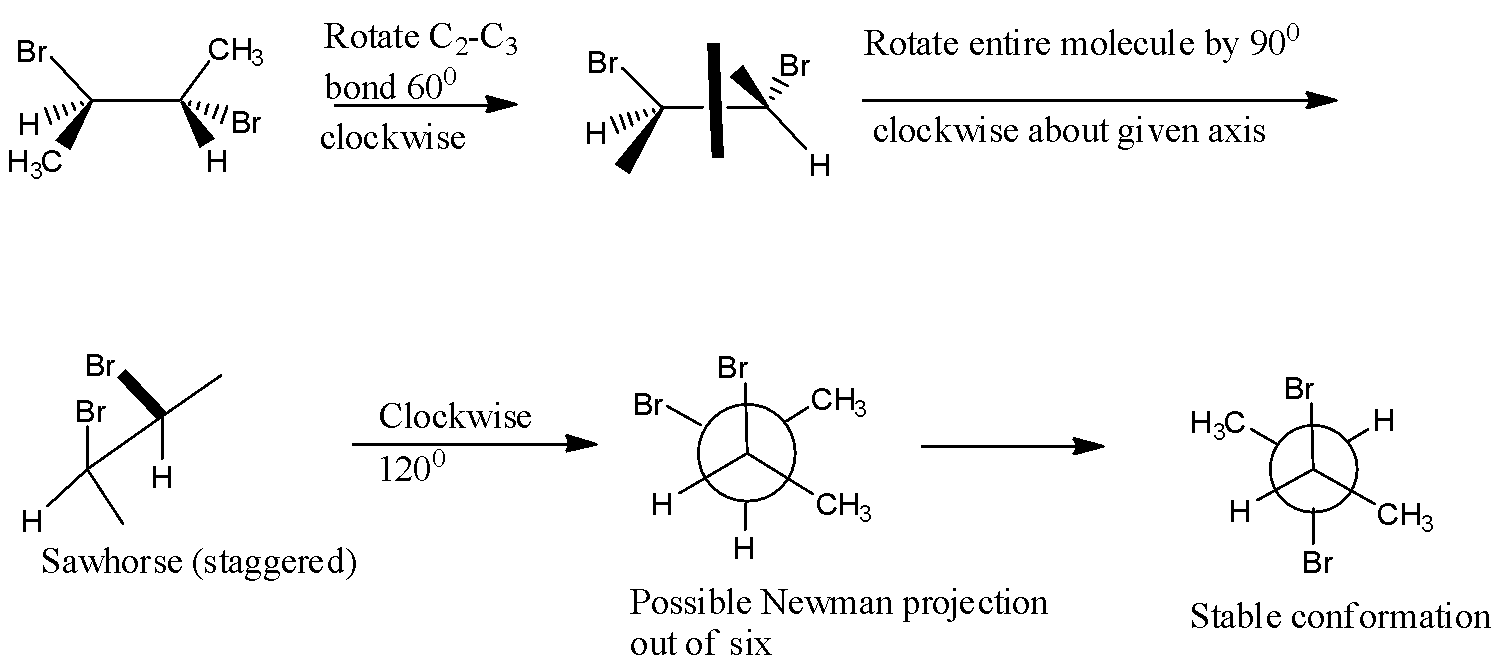
- The most stable conformation of 2R-3S-dibromobutane is the anti form of the compound where the two bromine atoms are exactly opposite to each other in the structural form.
- This is obtained stepwise by first rotating C2−C3 bond by 1200 counterclockwise and this gives the compound which when a mirror is placed in the plane , it bisects two stereocenters which shows that this is meso compound.
- The Newman projection which can be obtained by Sawhorse projection stepwise is as shown below,
Note: Note that meso form or meso isomer of a compound is the non – optically active member of a set of stereoisomers where at least two of which are equal which means that despite containing two or more stereogenic centres, the molecule is not chiral.
