Question
Question: How do you draw a Newman projection for hexane while sighting down \( {C_2} - {C_3} \) ?...
How do you draw a Newman projection for hexane while sighting down C2−C3 ?
Solution
For the given problem, we first need to figure out the normal structure of hexane which is a straight chain of six carbon atoms with all valances satisfied by the hydrogen atoms. Then figuring out the C2−C3 and then fixing them in Newman projection template. After fixing the groups on the template, we have the Newman structure of hexane.
Complete Step By Step Answer:
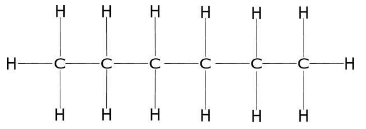
1. Draw the structure of hexane.
2. Convert the drawn structure from C2 and C3 to a wedge-dash structure.
3. Identifies the C2 and C3 groups.
The main chain of hexane is the horizontal zigzag line of carbon atoms. C1 is on the left.
The groups of C2 are H,H&CH3 . At C3 are H,H,&CH2CH2CH3 .
4. Draw the Newman projection template.
5. Attach the group to the carbon in the template.
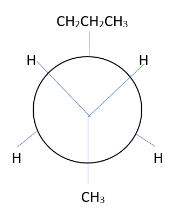
Shows the numerator from the left. The group of C2 is on the previous carbon atom. Place the CH3 group at the bottom. The two hydrogen atoms move to the other bond. The group of C3 moves to the rear carbon. The bulky CH2CH2CH3 group is at the top and two hydrogen atoms are on the other bond.
This is the most stable conformer because it has displaced volumetric methyl and propyl groups.
Note:
The Newman projection, useful in alkane stereochemistry, visualizes the structure of a chemical bond from front to back, with the front atom represented as a point and the rear carbon as a circle. The carbon atom in front is called near, while the atom behind is called far.
