Question
Question: How do you draw a chair conformation from a fischer projection?...
How do you draw a chair conformation from a fischer projection?
Solution
Chair conformation is the most accurate form of representation for mainly rings. It tells appropriately about the angle between the carbon atoms in a ring and also tells about the position of the group on each carbon atom present in a ring.
Complete step by step answer:
- Fisher projections are very appropriate for drawing long chain molecules which have a huge number of chiral carbon, for example carbohydrates.
- The fischer projection restricts the molecule into a two-dimensional plan, such that the absolute configuration of the molecule remains unchanged.
- Also, depending on the number of stereo centres in the molecule, the total number of stereoisomers present can be determined.
- Let us draw the Fischer projection of D-glucose. It is important to note that we need to change the position of the hydroxyl group on C-5 in the final orientation.
- This was important to understand the axial and equatorial bonds on carbon to determine which part goes up and which part goes down.
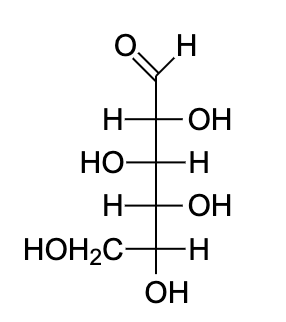
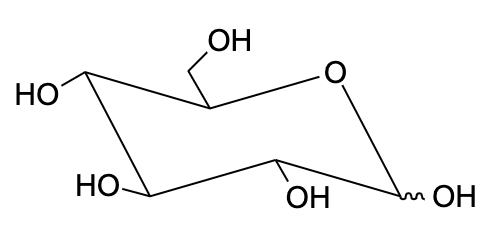
Note: The stability of chair conformation is found by adding up the A-value for each axial substituent. Lower the number, more stable the conformation is. The conformational isomers are the stereoisomers which differ in relative position of atoms within the molecule and which can be interconverted simply by rotation about the sigma bond. In this, the interconversion of the isomers does not require breaking and remaking of covalent bonds.
