Question
Question: How do you do additional reactions of alkenes?...
How do you do additional reactions of alkenes?
Solution
Alkenes are unsaturated molecules which will have at least one double bond in them, Always a molecule will tend to have the most stable state. An Additional reaction involves formation of new bonds.
Complete step-by-step answer: So in the question it is asked to discuss the additional reaction of the alkenes. We should first have an idea on what are alkenes, their stability and how they undergo reactions.
From the time we study about organic chemistry we deal with various classes of hydrocarbons, like alkanes, alkenes, alkynes etc.
The alkanes are saturated compounds, they only form single bonds between C atom and H atom.
The alkenes and alkynes are unsaturated compounds which have presence of multiple bonds in them.
Alkenes are the molecules which possess at least one double bond between carbon and H atoms. They have a general formulaCnH2n.
In an alkene molecule, in the double bond present, one is sigma bond and one is pi bond. The alkenes have stable configuration, but they are not the most stable one.
In the addition reaction of alkenes the conversion of a pi bond is occurring with two strong sigma bonds.
As the name states we add a reagent with the alkenes which reacts with the alkenes and replaces the multiple bond and forms two sigma bonds.
For more understanding, let us discuss the mechanism involved.
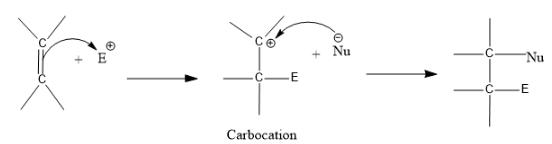
First the alkene will react with the species added, say an electrophile E is added, it reacts with alkene and forms a carbocation intermediate. And the carbocation will combine with a nucleophile present in the molecule and form the fiat product.
Note: Few reactions of alkenes are:
Hydration reaction in which a water molecule is added.
Hydrogenation reaction in which hydrogen molecule is added.
Halogenation reaction in which halogens are added and hydrohalogenation reactions in which hydrogen halides are added.
The addition reaction follows Markovnikov's rule of addition of reagent, but there are exceptions like HBr which does not obey the rule.
