Question
Question: How do you determine the enthalpy change for the reaction below using the enthalpy of combustion dat...
How do you determine the enthalpy change for the reaction below using the enthalpy of combustion data in the table:
3C(s)+4H2(s)→C3H8(s)?
| Substance | ΔHc⊕/kJmol−1 |
|---|---|
| C(s) | -394 |
| H2(g) | -286 |
| C3H8(g) | -2219 |
Solution
The enthalpy of the combustion is defined as the heat energy which has been given out when 1 mole of the given substance get burnt completely in oxygen. The combustion is a process or the reaction which is always exothermic in form. The enthalpy change for the reaction is always negative. So by convention we can conclude that the molar heat of the combustion given in the table is a positive value.
Complete step by step answer:
In this question we can calculate the enthalpy of the reaction by constructing the Hess cycle. The Hess law states that the change in the overall enthalpy of the reaction is independent of the taken route. This law tends to follow the first law of thermodynamics. The application of it lies in calculating the change in the enthalpy values which are very difficult in obtaining them through experimentally. This law is the consequence of the fact that the enthalpy is a function of state or a state function.
So the reaction is:
3C(s)+4H2(s)→C3H8(s)
Look at the following cycle to understand:
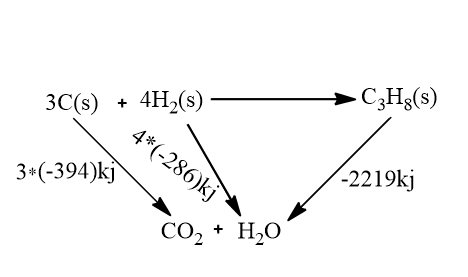
So the process of formation of propane through carbon and hydrogen and the formation of carbon dioxide and water is equal to the formation of carbon dioxide and water directly through carbon and hydrogen.
So we will get,
ΔH−2219=3(−394)+4(−286)
On solving we get,
ΔH=−107kJ
So the enthalpy change for the reaction is ΔH=−107kJ.
Note: The enthalpy of combustion is an exothermic process and its value is positive whereas the enthalpy of formation can be negative and positive. The standard enthalpy change of combustion is the amount of energy which is released when the substance undergoes complete combustion. This is the energy which has been released by 1 mole of the substance.
