Question
Question: How do you determine the acidity of aromatic compounds?...
How do you determine the acidity of aromatic compounds?
Solution
Acidity is defined as the tendency of a compound to release the Hydronium ion (H+) easily. We know that aromatic compounds are those which follow Huckel’s rule. Aromaticity plays a big role in determining the acidity of aromatic compounds.
Complete answer:
Aromatic compounds are those which fulfils the huckel’s rule. As per huckel an aromatic compound is one which contains (4n+2)π electrons in the ring. Where n= any whole number. Along with it, aromatic compounds should be planar and should show complete dissociation of π electrons.
We can determine the acidity of any aromatic compound by measuring its Ka. Alternatively, Ka may be also expressed as pKa which is defined as pKa=−logKa. Smaller the pKa value, stronger is the acid.
But we should remember that acidity or Ka values depend on various factors. Few are as follows.
1. Resonance: we know that in resonance the delocalization of the pie electrons takes place and as a result due to this the stability of the aromatic compound increases.
For example: Phenol is a resonance hybrid of following structures.
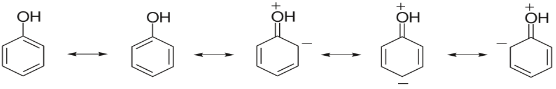
It is clear that three structures have positive charge on oxygen of the -OH group. This oxygen attracts the electron pair of OH towards itself, making it easier for the hydrogen to escape. And we know that if hydrogen can be easily escaped then the compound is more acidic.
Also, the phenoxide ion formed after the release of hydrogen is much more stable by resonance itself. And we know if the product formed is more stable then the reaction is favored. So, we can conclude that Phenol will be more acidic as the phenoxide ion formed after release of hydrogen is stabilized by resonance.
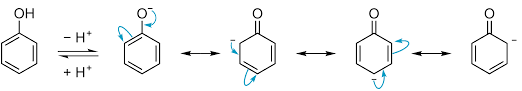
It should be noted that phenoxide ion is more stable than phenol as there is no charge separation in it, unlike phenol.
2. Electron withdrawing group: Such groups withdraw electrons from the aromatic ring, disperses the charge and as result makes the compound more acidic and thus increasing the value of Ka. Examples of such groups are NO2,CN,etc
3. Electron releasing group: Such groups, donates the electron in the ring thus intensifying the negative charge and as a result decreases the acidity of the compound. Examples of such groups are −NH2,−OR,−R
Note: It should be noted that while determining the acidity of the compound all the above factors should be kept in mind. Also, if more than one electron withdrawing or donating group is attached then always consider the more stronger group.
