Question
Question: How do you determine if a molecule is nonpolar \(?\)...
How do you determine if a molecule is nonpolar ?
Solution
Non-polar molecules are the ones in which the electrons are shared equally between the nuclei, as a result the distribution of charge is even and the force of attraction between different molecules is small. So we need to determine whether the electrons are shared equally between the atoms in a molecule in order to find whether it is non-polar.
Complete step-by-step answer:
First, draw the Lewis Dot structure for the given compound. Then identify each bond as either polar or nonpolar. If the difference in electronegativity between the atoms in a bond is greater than0.5, then we consider the bond to be polar but if the difference in electronegativity between the atoms is less than 0.5, then the bond is considered to be nonpolar.
Now, if there are no polar bonds, the molecule is nonpolar, but if the molecule has polar bonds then proceed further. If there is only one central atom present in the given molecule, then examine the electron groups around it. If there are no lone pairs of electrons present on the central atom, and if all the bonds to the central atom are the same, the molecule is nonpolar. But if the central atom has at least one polar bond and if the groups bonded to the central atom are not all identical, then proceed further
Draw a geometric sketch of the molecule and determine the symmetry of the molecule using the following steps. Describe the polar bonds with arrows, with the arrow head pointing toward the more electronegative element. Use the length of the arrow to show the relative polarities of the different bonds. Then decide whether the arrangement of arrows is symmetrical or asymmetrical. If the arrows are of different lengths, and if they do not balance each other, the molecule is polar and if the arrangement is asymmetrical, the molecule is polar. But if the arrangement is symmetrical and the arrows are of equal length, the molecule is nonpolar.
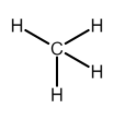
For example, Methane (CH4) has a net electronegativity difference of 0.4, also it is symmetrical and hence has nonpolar covalent bonds.
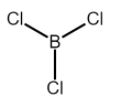
But Boron trichloride (BCl3) has a net electronegativity difference of 1.0 so it has polar covalent bonds. However, it is symmetrical, thus it is non-polar in general but has polar covalent bonds.
Note: Proceed in a stepwise manner if you jump steps then the interpretation can be wrong. Lewis Dot structure must be correct. Also remember that a molecule may be nonpolar but it may have polar covalent bonds. Hence do not confuse between the polar bonds and polar molecules.
