Question
Question: How do you determine if a compound is meso when trans or cis is not listed? Such as in the example o...
How do you determine if a compound is meso when trans or cis is not listed? Such as in the example of 2,3− pentanediol.
Solution
Trans and cis isomers are the pair of molecules which have same molecular formula but differ from each other on the basis of the orientation of the functional group. They differ orientation in three dimensional spaces. They both are stereoisomers.
Complete step-by-step answer: Cis isomers are defined as the compound whose functional groups are attached in the same side of the double bond. Trans isomers are defined as the compound whose functional group is attached in the opposite direction of the double bond.
Meso compounds consist of two chiral centres and one side and other of the mirror plane are mirror images of each other.
To determine if a compound is meso when trans and cis is not listed, firstly we should draw stereo chemical formulas of the isomers and then see whether they are super imposable mirror images or not.
Let us take an example of 2,3− pentanediol,
In order to have cis or trans isomers we should must see whether the molecule has double bond or ring like structure. The molecule shows restricted rotation when a molecule has a double bond or ring-like structure. 2,3− pentanediol neither have a ring like structure nor a double bond this means they do not contain any cis or trans isomers.
Now let us see the fischer projection of these isomers-
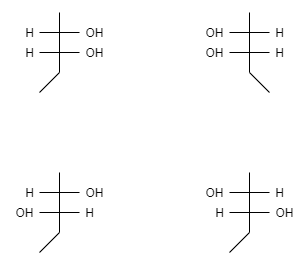
These isomers do not have superimposable mirror images of each other and therefore they are said to be a meso compound.
Note: In this question, we have discussed the meso compounds that show super imposable mirror images of each other. If a compound have double bond and ring like structure then it will behave as cis and trans isomers.
Fischer projection gives us a three dimensional structure of a molecule in which the stereo centres are represented as a cross.
