Question
Question: How do you convert the following? Toluene to Benzoic acid...
How do you convert the following?
Toluene to Benzoic acid
Solution
Hint : We must know that the oxidation of toluene using suitable oxidizing agent will give you benzoic acid.Toluene,also known as methylbenzene and its chemical formula is C7 H8 whereas Benzene’s chemical formula is C6 H6.
Complete step by step solution :
We can prepare benzoic acid from toluene by laboratory process.
Therefore, Mix 3 ml of Toluene with 10 gm of Potassium Permanganate and 20 ml of dilute solution of sodium hydroxide in round bottom flask
Set up the reflux and start mixture to reflux for 3 to 4 hours until oily toluene disappears.
Cool down the solution and filter out the manganese oxide from the solution.
Add concentrated HCl to the filtrate
Collect the precipitate of benzoic acid and recrystallize using hot H2O, or can extract the benzoic acid with ether.
We can write the above process in chemical equation form as below.
Toluene should be heated in presence of alkaline KMnO4 to carry out oxidation of Toluene. This process oxidizes toluene into potassium benzoate ions.
Potassium benzoate ions are then followed by acidification to form benzoic acid.
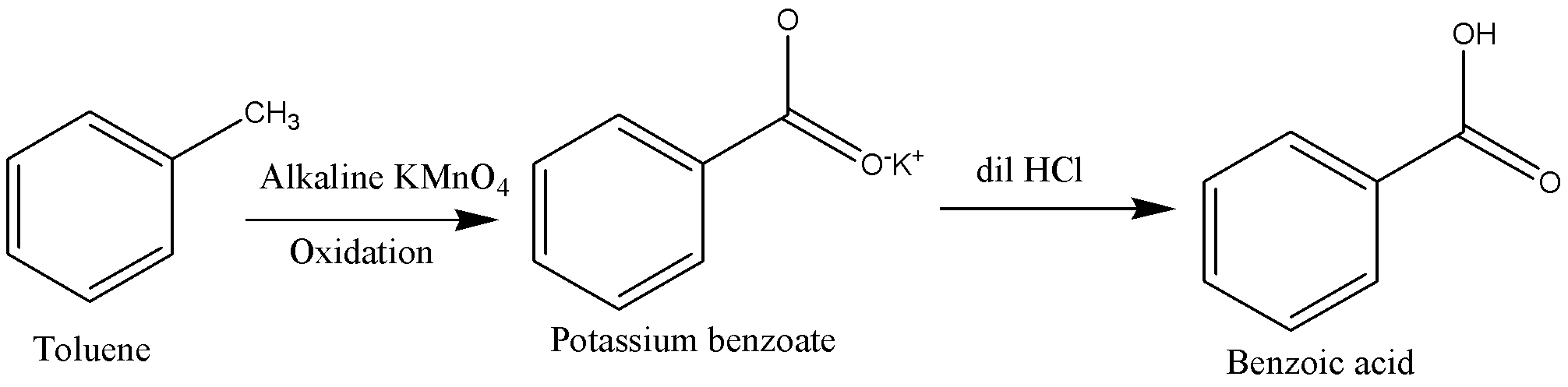
We will reagents such as acidic potassium permanganate (KMnO4), Acidic potassium dichromate (K2Cr2O7) and diluteHNO3. Other oxidizing agents also can use in conversion of toluene into benzoic acid.
This is a simple oxidation method where the Methyl group gets converted to a carboxylic group using potassium permanganate as an oxidizing agent. Potassium hydroxide provides an alkaline medium because strong oxidizing agents are stable only under alkaline medium. HCl addition neutralizes the added KOHand then converts potassium benzoate in the filtrate into benzoic acid.
Note : Even though there are many different ways to prepare Benzoic acid from Toluene, the simple one is mentioned above.Under neutral conditions, Mn+7is reduced to Mn+4and under acidic conditions it is reduced toMn+2. Both neutral and acidic medium conditions make KMnO4less powerful oxidizing agents.
