Question
Question: How do you calculate the dipole moment of water?...
How do you calculate the dipole moment of water?
Solution
Dipole moment is a vector quantity means it has magnitude and direction. Dipole moment is a measure of polarity of chemical bonds present in between two atoms in a particular molecule. In dipole moment the separation of negative and positive charge takes place.
Complete answer:
- In the question they asked how we can calculate the dipole moment of water.
- There is a formula to calculate the dipole moment and it is as follows.
Dipole moment (μ) = charge (Q) × distance of separation (r)
- Now coming to the calculation of the dipole moment of the water molecule.
- We know that the electrons are localized around the oxygen atom in the water molecule.
- The localization of electrons is due to the high electronegativity of the central oxygen atom in water molecules.
- Due to the presence of lone pairs of electrons on the oxygen molecule the shape of the molecule is going to be a bent shape.
- The shape and charge separation in the water can be seen in the following picture.
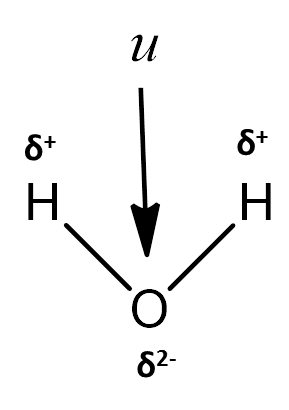
- The bond angle which is present in water is 104.5o .
- The individual bond moment of each hydrogen-oxygen bond is 1.5 D.
- There are two hydrogen in water molecules, the two hydrogen creates their individual dipole moments.
- We have to calculate the individual dipole moments and later we have to do the sum to get the dipole moment of the water molecule.
- The dipole moment caused by the left hydrogen in water molecule = 1.5D×cos(52.2388o)=15D×0.612=0.9187
(Here, 52.2388o=2104.5o )
- The dipole moment caused by the right hydrogen in water molecule = 1.5D×cos(52.2388o)=15D×0.612=0.9187
(Here, 52.2388o=2104.5o )
Therefore the net dipole moment of the water molecule = 0.9187+0.9187=1.837D.
Note: The unit to measure dipole moment of the molecules is D (Debye). The dipole moment for linear molecules like beryllium difluoride are 0. Because the dipole moment caused by individual fluorine atoms is zero.
