Question
Question: How do you balance this redox reaction, using the oxidation number method? \(HN{{O}_{3}}(aq)+{{C}_...
How do you balance this redox reaction, using the oxidation number method?
HNO3(aq)+C2H6O(l)+K2Cr2O7(aq)→KNO3(aq)+C2H4O(l)+H2O(l)+Cr(NO3)3(aq)
Solution
The oxidation number method is the method of balancing the equation by equating the oxidation number of the elements. First, you have to balance all the elements except Hydrogen and Oxygen. In the last hydrogen and oxygen are balanced by adding water molecules.
Complete answer: We have to balance the given chemical equation using the oxidation number method, so there are some rules that must be followed. First, we have to write the skeletal equation given in the question. The equation is:
HNO3(aq)+C2H6O(l)+K2Cr2O7(aq)→KNO3(aq)+C2H4O(l)+H2O(l)+Cr(NO3)3(aq)
Now, we have to write all the oxidation numbers on the elements present in the equation. These are represented below:

From all the oxidation numbers mentioned in the equation above, we can see that, the oxidation number of carbon changes from -2 to -1, and the oxidation number of chromium changes from +6 to +3.
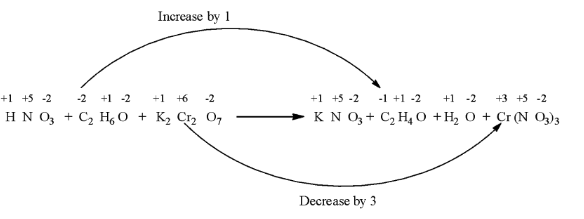
Now we have to balance the oxidation numbers on both the side: multiply C2H6O and C2H4O with 3, multiply Cr(NO3)3 with 2 because there is only chromium atom in this compound.
The equation will be:
HNO3(aq)+3C2H6O(l)+K2Cr2O7(aq)→KNO3(aq)+3C2H4O(l)+H2O(l)+2Cr(NO3)3(aq)
Carbon and chromium are balanced in this equation, so now let us balance the potassium atom. There are 2 potassium atoms on the reactant side and 1 on the product side.
HNO3(aq)+3C2H6O(l)+K2Cr2O7(aq)→2KNO3(aq)+3C2H4O(l)+H2O(l)+2Cr(NO3)3(aq)
Now, let us balance the nitrogen atoms, there is one nitrogen atom on the reactant side and 8 nitrogen atoms on the product side.
8HNO3(aq)+3C2H6O(l)+K2Cr2O7(aq)→2KNO3(aq)+3C2H4O(l)+H2O(l)+2Cr(NO3)3(aq)
Now, let us balance the oxygen atoms, by adding 6 molecules of water molecules on the product side.
8HNO3(aq)+3C2H6O(l)+K2Cr2O7(aq)→2KNO3(aq)+3C2H4O(l)+7H2O(l)+2Cr(NO3)3(aq)
In this equation all the atoms are balanced, so this is the balanced equation.
Note: If the equation is acidic then you have to balance the hydrogen atom by hydrogen ions (H+), if the equation is basic then you have to balance the hydrogen atom by hydroxyl ions (OH−)
