Question
Question: How do we get ammonia?...
How do we get ammonia?
Solution
The ammonia is a chemical with a number of uses, in the textile industry, fermentation industry, household, laboratory etc.
It has a pungent smell and is colourless liquid which is mildly basic in nature, and is dangerous when used in concentrated form.
Complete step-by-step answer: The structure of ammonia contains one atom of nitrogen and three atoms of hydrogen and it has a characteristic pungent smell. When added to water, it forms ammonium hydroxide which makes the solution basic. The structure can be represented as,
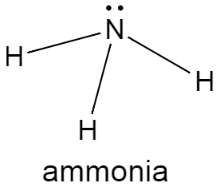
Ammonia can be prepared in large quantities by the use of Haber’s process which involves direct combination of hydrogen and nitrogen under the conditions of high pressure and this process requires the presence of a catalyst. The reaction can be represented as,
N2+H2→NH3
Here as we can see the nitrogen and hydrogen combine in order to form ammonia.
Ammonia can also be made in the laboratory by using certain chemicals and performing required steps. First we need to heat an ammonium salt, let say we take ammonium chloride, along with a strong alkali, like calcium hydroxide or sodium hydroxide. The reaction can be represented as,
2NH4Cl+Ca(OH)2→CaCl2!! !!+2H2O+2NH3(g)
Here in the reaction we can see that ammonium chloride reacts with calcium hydroxide in order to form calcium chloride and water as a side product along with ammonia.
Additional information:
Ammonia has many uses, as it could be used as a fertilizer which increases the yield of the plants or crops. Ammonia is also used as a cleaner where water and ammonia is mixed and used to clean glass and steel. It has an application as an antimicrobial agent. It could be used as a refrigerant.
Note: Ammonia can be made in the laboratory by heating ammonium salt along with some alkali like sodium or potassium hydroxide.
Ammonia is prepared on a large scale in the industries by the help of Haber’s process where they use direct combination of nitrogen and hydrogen in order to make ammonia.
