Question
Question: How do water molecules act like "little magnets"?...
How do water molecules act like "little magnets"?
Solution
To answer this question we have to understand the structure and the polar nature of water molecules. Water is used as solvent for most of the reactions and it also plays a vital role in living organisms.
Complete answer:
Water is a simple molecule which consists of one oxygen atom bonded to two hydrogen atoms. The chemical formula of water is H2O. Firstly, the structure of water was predicted to be linear but later with help of VSEPR theory it was found that the structure of water is bent. The structure of H2O is bent because of the presence of two lone pairs on oxygen.
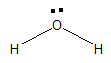
The oxygen is more electronegative than hydrogen atom. So, the oxygen atom possesses a partial negative charge and the hydrogen atom possesses positive negative charge. Therefore, water is polar in nature.
Since it is polar in nature the oxygen atom which possesses partial negative (−δ) can attract protons which possess positive charge or any element or atom that possess positive charge on it.
Same goes with hydrogen, which possesses partial positive charge (+δ) and can attract oxygen in other water molecules or any other atom that possesses a negative atom.
Basically, when a magnet is brought nearer to another magnet it attracts each other. Unlike charges, they attract each other. This is the same that happens in case of Water molecules too.
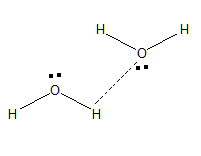
This is the reason why the water molecule is said to act like a little magnet.
Note:
The polarity of water molecules the point that accounts for its mechanism as a little magnet. Since water molecules can form clusters as illustrated above they are used as solvent for many reactions. If the structure of the water molecule is linear the dipole moment in the water molecule will be 0. Since the water molecule has a bent structure it possesses some dipole moment.
