Question
Question: How do the structure of atoms of hydrogen and helium differ?...
How do the structure of atoms of hydrogen and helium differ?
Solution
We need to know the position of the given atoms in the periodic table. Here position basically refers to the atomic number of the atoms. Atomic numbers will tell us the number of electrons present in the atom. Number of electrons will determine the structure of the atoms. Although there are other factors which determine the structure of an atom, the basic difference between the structures of two given atoms can be found by observing their number of electrons.
Complete step by step answer:
In the modern periodic table, a clear trend of atomic size is visible which depends on the following factors:
The number of protons in the nucleus which are positively charged and concentrated in the nucleus (called the nuclear charge).
The number of energy levels called shells holding electrons and the number of electrons in the outer energy level called valence electrons
The number of electrons held between the nucleus and its outermost electrons (called the shielding effect).
Keeping these in mind, the difference between the two elements are as follows:
| Hydrogen| Helium
---|---|---
1.| The atomic number of the hydrogen is 1.| The atomic number of helium is 2.
2.| The number of electrons in the hydrogen atom is 1.| The number of electrons in the helium atom is 2.
3.| It has the smallest atomic size since it is the first element of the periodic table.| It has the second smallest atomic size after hydrogen
4.| It has one proton and one neutron concentrated at its nucleus.| It has two protons and two neutrons concentrated at its nucleus.
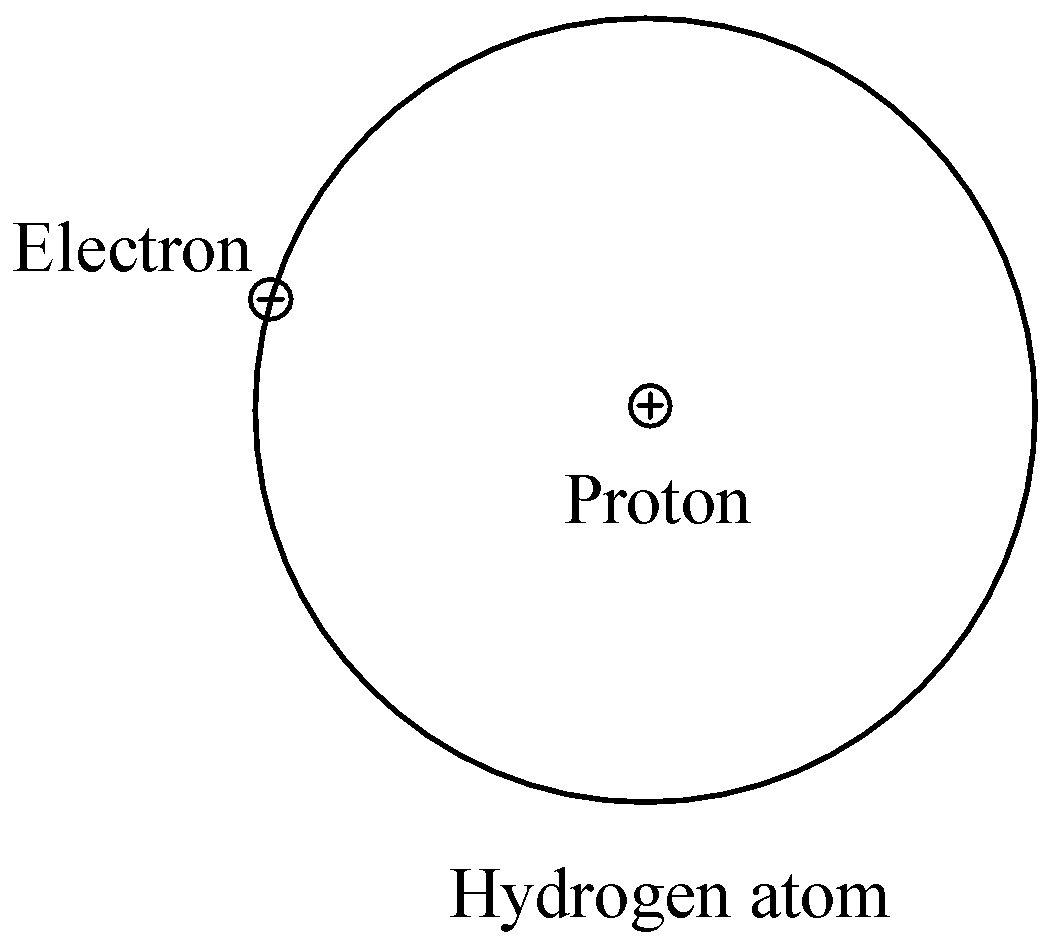
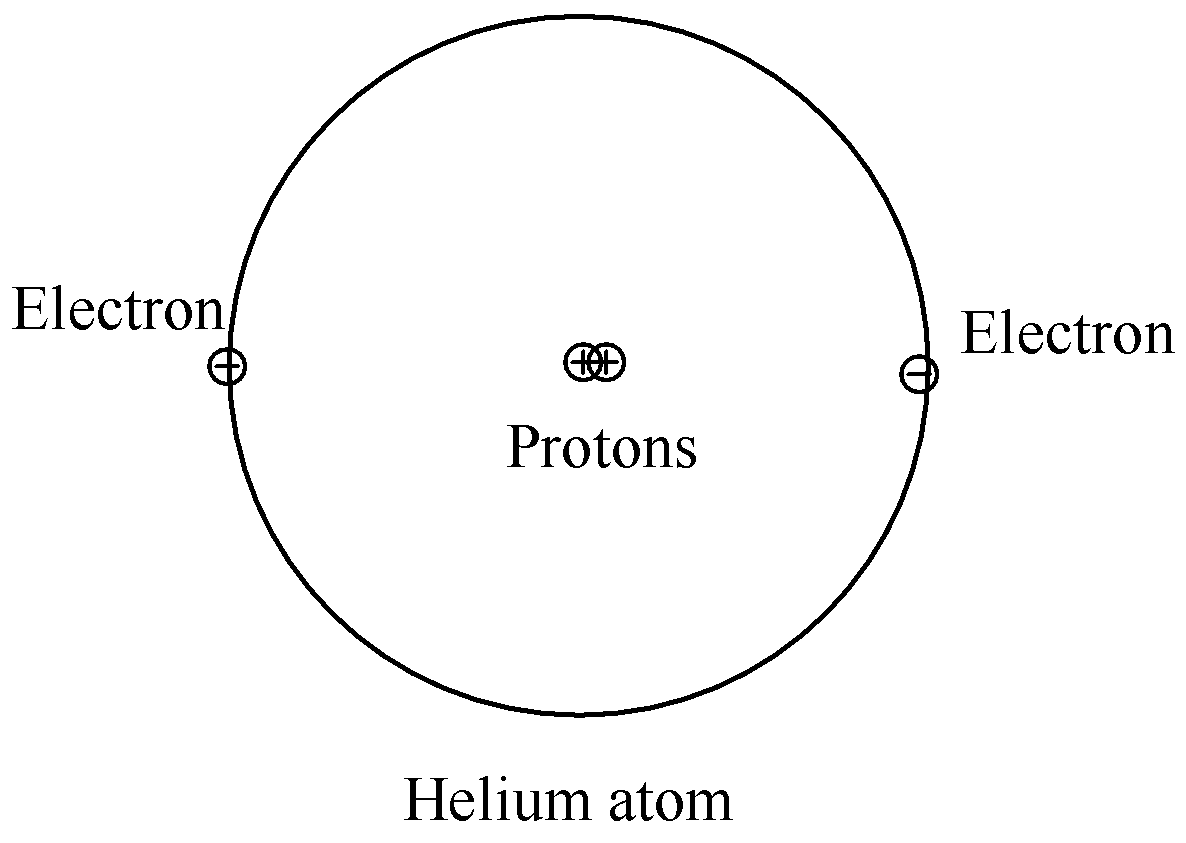
To conclude, hydrogen and helium are the first two elements of the periodic table. The atomic numbers of hydrogen and helium are 1 and 2 respectively. Due to its few number of electrons, the above factors are not to be taken into consideration since it applies to complex atoms having a greater number of electrons. Since helium has 1 electron more than hydrogen, it is obvious that it will have a bigger atomic size than that of hydrogen.
Hence, the structure of atoms of hydrogen and helium differ by the fact that helium has a bigger atomic size than that of helium.
Note: We must be noted that Rutherford’s Atomic Theory was the first experiment that provided scientists with an approximate measurement for the size of the atom. The atomic size is defined as the distance from the nucleus to the valence shell. Rutherford was able to determine quantitatively that the nucleus had a radius size smaller than 3×10−12cm . Also, atomic size increases as we go down a group in the periodic table.
