Question
Question: How do I draw the axial and equatorial bonds in a cyclooctane?...
How do I draw the axial and equatorial bonds in a cyclooctane?
Solution
A cyclooctane is a saturated hydrocarbon that has an eight-membered simple ring structure and has the molecular formula C8H16 or (CH2)8. It exists in various different conformations like boat-chair, crown, tub, boat-boat, twist boat-chair, and twist chair-chair. The most stable among these conformations is the boat-chair conformation.
Complete answer: To draw the axial and equatorial bonds of a compound, we first need to know what axial and equatorial bonds are.
Now, we know that in a cycloalkane, each carbon atom forms four bonds, two with the other carbon atoms of the ring, and the other two with the non-ring atoms, like hydrogen. The carbon bond to the non-ring atoms can be either axial or equatorial.
Axial bonds are the bonds that form an 90∘ angle with the ring plane whereas equatorial bonds are the bonds that only make a small angle with the plane.
When a corner is pointing up, the axial bonds are drawn straight up, and when the corners are pointing down, the axial bonds are drawn straight down.
Equatorial bonds are either drawn up and out, or down and out.
There are few points that need to be remembered while drawing an axial or equatorial bond.
- Every carbon atom has both an equatorial bond and an axial bond.
- When a substituent is added and replaces the hydrogen atom, the cycloalkane ring will flip so that the substituent will be in an axial position in one conformation and the equatorial position in the different conformation.
Now, we know that the most stable conformation of cyclooctane is the boat-chair conformation.
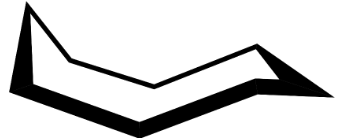
So, the equatorial and axial bonds will be drawn as:
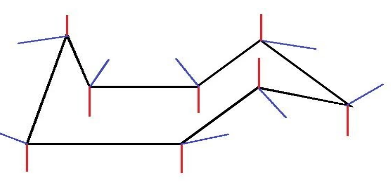
Here, the equatorial bonds are represented in blue and the axial bonds are represented in red.
Note: It is very easy to confuse between the two and hence should be noted that when talking about the stereochemistry of a ring structure, the terms cis and trans are not linked to the terms equatorial and axial directly. The two naming systems should be tried to inter-convert directly.
