Question
Question: How do I calculate the bond order of \( H_2^ + \) and \( H_2^ - \) ?...
How do I calculate the bond order of H2+ and H2− ?
Solution
Hint : To calculate the bond order of a diatomic species, we’ll have to refer to its Molecular orbital Diagram. The MO Diagram of Hydrogen Molecule consists of one Bonding and one Anti Bonding Orbitals.
Complete Step By Step Answer:
We are given two species: H2+ and H2− . The bond order is defined as the difference between the number of electrons in Bonding MO (B) and antibonding MO (AB). The formula can be given as:
Bond Order=21(B−AB)
Consider a neutral Hydrogen Molecules which consists of 2 electrons in total. The MO diagram is drawn as follows.
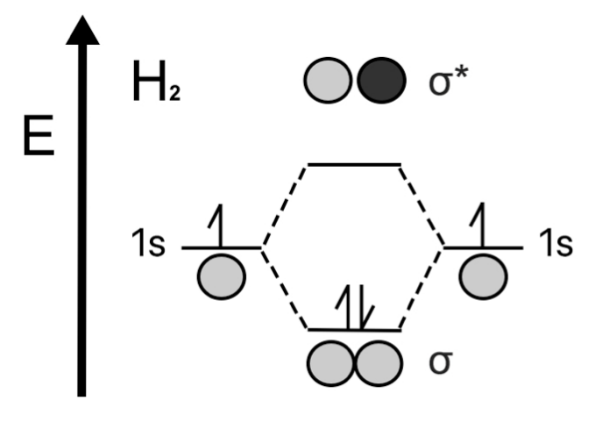
The bond order of neutral Hydrogen Molecule is =21(2−0)=1
Hence it has a single bond between two hydrogen atoms.
In H2+ one atom is lost, which means that it has only one electron left. That electron will be in the bonding MO. The bond order of this molecule is =21(1−0)=0.5
The bond order of H2+ is 0.5 .
In H2− one electron is gained. Hence there are two electrons in bonding and one in antibonding MO. Total three electrons present in total. The bond order can be calculated as =21(2−1)=0.5
The bond order of H2− is also 0.5 .
The bond order of H2− and H2+ is the same. In H2+ the electrons are present in the bonding MO only, hence it’ll be more stable and will have higher energy than H2− . In H2− there is one electron in the antibonding MO, hence the energy and stability are reduced.
Note :
Bond order is inversely proportional to bond length. Higher the bond order, the distance between the atoms will be less, hence the Bond length will reduce. But bond order and bond energy are directly proportional. The more the bond order, the more the Bond energy.
