Question
Question: How do Grignard reagents react with alcohols?...
How do Grignard reagents react with alcohols?
Solution
Hint : Grignard reagent: It is a very useful organometallic compound which consists of magnesium atoms bonded to a halogen and an alkyl group. General formula of Grignard reagent is R−Mg−X . It is used in various specific tests to determine functional groups of unknown compounds.
Complete Step By Step Answer:
The haloalkane when react to magnesium in the presence of vinyl or aryl halides, formation of a very important organometallic compound i.e., Grignard reagent takes place as follows:
The mechanism for the formation of Grignard reagents is as follows:
Step-1: Homolytic cleavage of R−X bond takes place in haloalkanes.
R−X→R∙+X∙
Step-2: Attack of halogen radical on magnesium atoms.
X∙+Mg→∙MgX
Step-3: Attack of R∙ on the product formed in step-2.
R∙+∙MgX→R−Mg−X
Grignard reagent is a good nucleophile as well as a strong base, so it can react with compounds having functional groups like epoxide, ketone, aldehydic, and alcohol. When alcohols react with Grignard reagent, then the acid-base reaction takes place and formation of magnesium alkoxide and respective alkane takes place. The reaction is as follows:
R−OH+R′MgX→R−OMgX+R′−H
Hence Grignard reagent reacts with alcohols to form respective alkanes.
Additional Information:
Reaction of Grignard reagent with compounds having carbonyl groups to give alcohol as a product:
Grignard reagent attacks at carbonyl centres of the compounds to form an intermediate which on further reaction forms alcohol and hydroxy-magnesium halide. The reaction proceeds as follows:
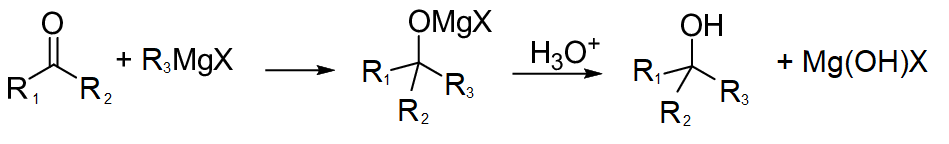
Note :
Grignard reagent acts as both a nucleophile as well as a base. But at priority, it will act as a good base rather than nucleophile because acid-base reactions are very fast as compared to other reactions.
