Question
Question: How do electrons fill orbitals?...
How do electrons fill orbitals?
Solution
The central structure of a particle is the nucleus, which contains protons and neutrons. This nucleus is encircled by electrons. In spite of the fact that these electrons all have a similar charge and a similar mass, every electron in a molecule has an alternate measure of energy. Electrons with the most minimal energy are discovered nearest to the nucleus, where the attractive power of the positively charged nucleus is the highest. Electrons that have higher energy are discovered further away.
Complete step by step solution:
The sequence in which orbitals are filled is given by the Madelung rule. The rule depends on the total number of nodes in the nuclear orbital, n+l which is identified with the energy. In this specific situation, n stands for the primary quantum number and l stands for the azimuthal quantum number. The qualities l=0,1,2,3 compare to the s,p,d,f names, individually. As per the guideline, electrons fill orbitals beginning at the most minimal accessible energy states prior to filling higher states (e.g., 1s preceding 2s ).
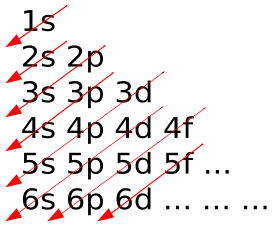
An Aufbau diagram utilizes arrows to portray electrons. When there are two electrons in an orbital, the electrons are called an electron pair. Electron sets appear with arrows pointing in inverse ways. As indicated by the Pauli Exclusion Principle, two electrons in an orbital won't spin in a similar way. That is, an Aufbau chart utilizes arrows pointing in inverse ways. An arrow facing up means an electron turning one way and an arrow pointing downwards indicates an electron spinning in an alternate way. In the event that the orbital just has one electron, this electron is called an unpaired electron.
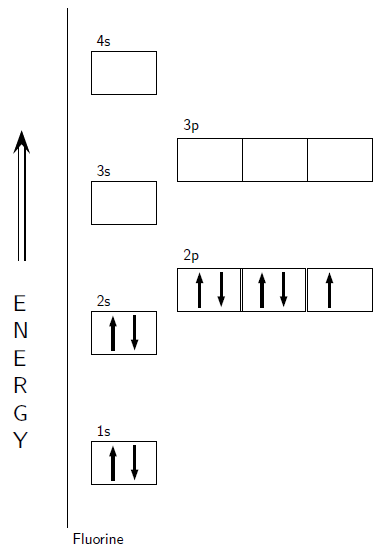
Note:
The Aufbau principle depends on the possibility that the sequence of orbital energies is fixed—both for a given component and between various components. This supposition is roughly evident—enough for the rule to be helpful—yet not genuinely sensible. It displays nuclear orbitals as "boxes" of fixed energy into which at most two electrons can be set. However, the energy of an electron in an atomic orbital relies upon the energies of the relative energy of different electrons of the atom.
