Question
Question: How do anhydrides react with water?...
How do anhydrides react with water?
Solution
We know that acid anhydride is a functional group in which a hydrogen atom joins two acyl groups. If the acid anhydride is obtained from two different carboxylic acids, then the acid anhydride is termed as mixed anhydride.
Complete step by step answer:
Let’s understand the structure of anhydride with the help of a compound.
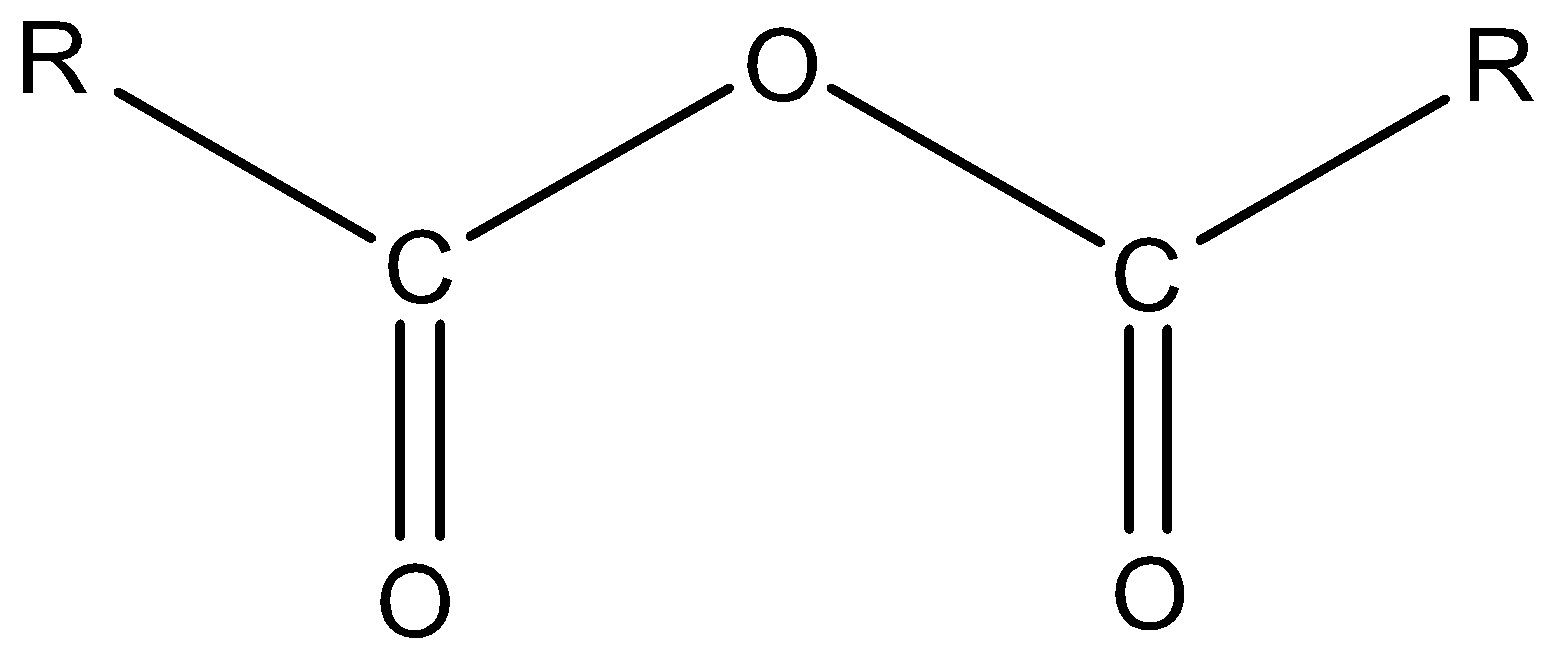
Here, the R represents alkyl groups. Both the alkyl groups may be the same or different. If the alkyl groups are different, then it is mixed anhydride.
Let’s discuss the reaction of anhydrides with water.
The reactivity of Acid anhydrides is less than the acid chlorides. The hydrolysis reaction of acid anhydrides is slow. This reaction produces two molecules of carboxylic acid.
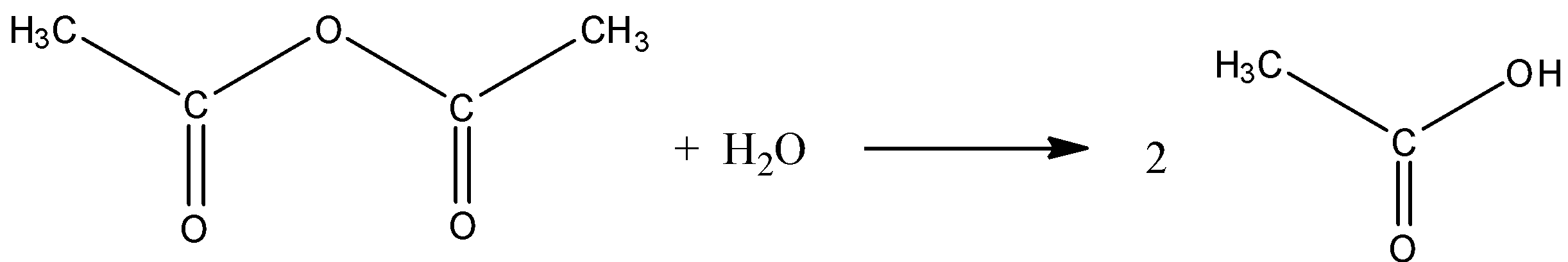
Additional Information:
Let’s discuss some other chemical properties of acid anhydride.
- Acid anhydrides when reacts with phenols and alcohols, produces esters.
(CH3CO)2O+C2H5OH→CH3COOC2H5+CH3COOH - Acid anhydrides can be reduced by lithium aluminium hydride to produce primary alcohols. In case of mixed anhydrides, two types of alcohol are produced.
- When anhydride reacts with aromatic hydrocarbons in the presence of aluminium chloride, aromatic ketone forms.
Let’s discuss how to name an acid anhydride. The word ‘acid’ should be changed to ‘anhydride’ in case of both IUPAC and common name. For example ethanoic anhydride, etc.
Note: It is to be noted that in inorganic chemistry an acid anhydride is a molecule that forms acidic solutions in water. All acid anhydride are non-metals but all non-metals are not acid anhydride. For example, carbon monoxide is not an acid anhydride even though it is an oxide of carbon as it does not react with water.
