Question
Question: How can you tell the difference between an ester, ketone, carboxylic acid, aldehyde, amines, amides ...
How can you tell the difference between an ester, ketone, carboxylic acid, aldehyde, amines, amides and phenol?
Solution
Let that be ester, ketone, carboxylic acid, aldehyde, amines, amides or phenol; each one of them is just the additional part to a molecule hampering its general physical and chemical properties. But each one of them added to the molecule defines new properties different from each other.
Complete answer:
Let us see all of them one by one;
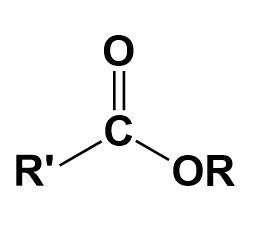
Ester-
A chemical compound in which at least one hydroxyl group is replaced by an alkoxy group.
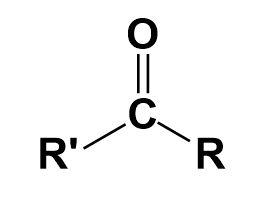
Ketone-
It is a functional group attached to a molecular chain. It contains a carbonyl group.
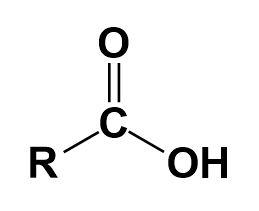
Carboxylic acid-
It is an organic acid that contains a carboxyl group. They occur widely and the most common ones are amino acids and fatty acids.
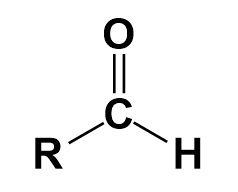
Aldehyde-
It is a functional group with the structure -CHO, consisting of carbonyl in the centre.
Amines-
These are the organic compounds that contain nitrogen atoms with a lone pair of electrons.
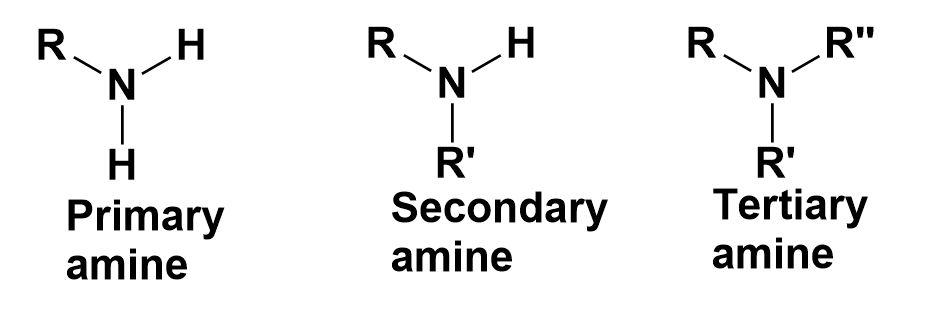
Amides-
They are the organic compounds that contain a functional group consisting of an acyl group linked to a nitrogen atom.
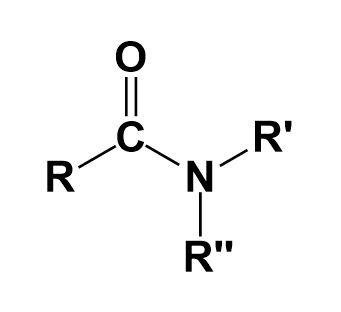
Phenol-
It is an aromatic organic compound, basically we can consider it as an alcohol. It consists of a phenyl group bonded to the hydroxyl group.
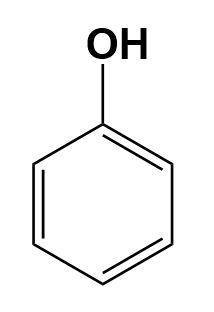
Now, by looking at the above diagrams we can find the differences easily.
Note:
During old times, scientists used to use Infra-red spectroscopy to determine the differences between the above given compounds. Modern methods include X-ray crystallography and NMR spectroscopy.
