Question
Question: How can you identify primary alcohol?...
How can you identify primary alcohol?
Solution
If the hydroxyl group is attached to the primary carbon atom, or at the end of a carbon chain, the alcohol formed is known as a primary alcohol. It has the functional group −CH2OH. Ethanol and propan-1-ol are examples of primary alcohol.
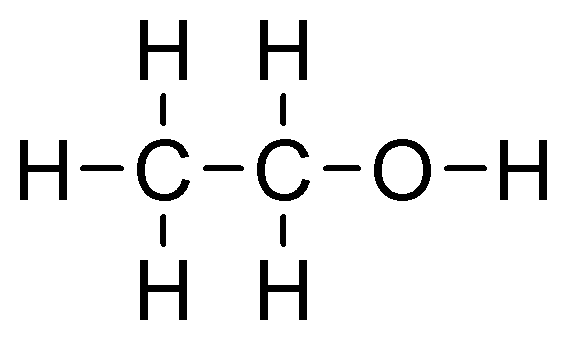
ethanol
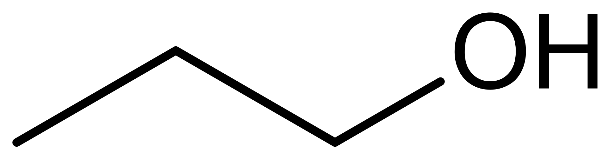
prop-1-ol
Complete answer:
There are different tests that can be used to identify primary alcohols and differentiate them from secondary and tertiary alcohols.
1. Lucas Test
This test is based on the different rate of reactivities of primary alcohols, secondary alcohols and tertiary alcohols. When an alcohol is treated with Lucas reagent, which is a mixture of concentrated hydrochloric acid and zinc chloride, turbidity is produced as the products formed are immiscible.
R−OHHClZnCl2R−Cl+H2O
The difference in time taken to achieve turbidity can be used to differentiate between the alcohols
- Primary alcohols do not produce turbidity at room temperature. An oily layer is produced on heating.
- Secondary alcohols take some time to produce turbidity as well as the oily layer.
- Tertiary alcohols produce turbidity immediately.
2. Oxidation Test
When the alcohols are oxidized with Na2Cr2O7 or sodium dichromate, the rate of oxidation is different for primary alcohols, secondary alcohols and tertiary alcohols.
The difference in rate of oxidation can be used to differentiate between the alcohols.
- Primary alcohols first oxides to form aldehydes and then are further oxidized to form carboxylic acids.
- Secondary alcohols are easily oxidized to form ketones and no further oxidation takes place.
- Tertiary alcohols do not get oxidized.
Note:
One interesting difference that can be noted in the physical properties of alcohols is that primary alcohols usually have higher boiling points and secondary and tertiary alcohols. This is because the (-OH) group in primary alcohols is more freely attached and hence will be able to interact more resulting in higher boiling point.
