Question
Question: How can you explain that \(CC{{l}_{4}}\) has no dipole moment?...
How can you explain that CCl4 has no dipole moment?
Solution
The dipole moment is identified by the polarity of the molecule and the direction of the dipole points from negative charge towards the positive charge. Find out if the given molecule is polar or not.
Complete step by step answer:
- In the previous chapters of chemistry, we have studied about the polarity and also about the measurement of polarity of the chemical compounds. We have also come across the terminologies like dipole moment and also about its identification.
Let us see if the given molecule is polar or not so that we can prove that CCl4 has no dipole moment.
- The electric dipole moment that is vector quantity is directed along the line from negative charge towards the positive charge. These dipole moments tend to point towards the direction of the surrounding electric field.
- Carbon tetrachloride has the structure as shown below:
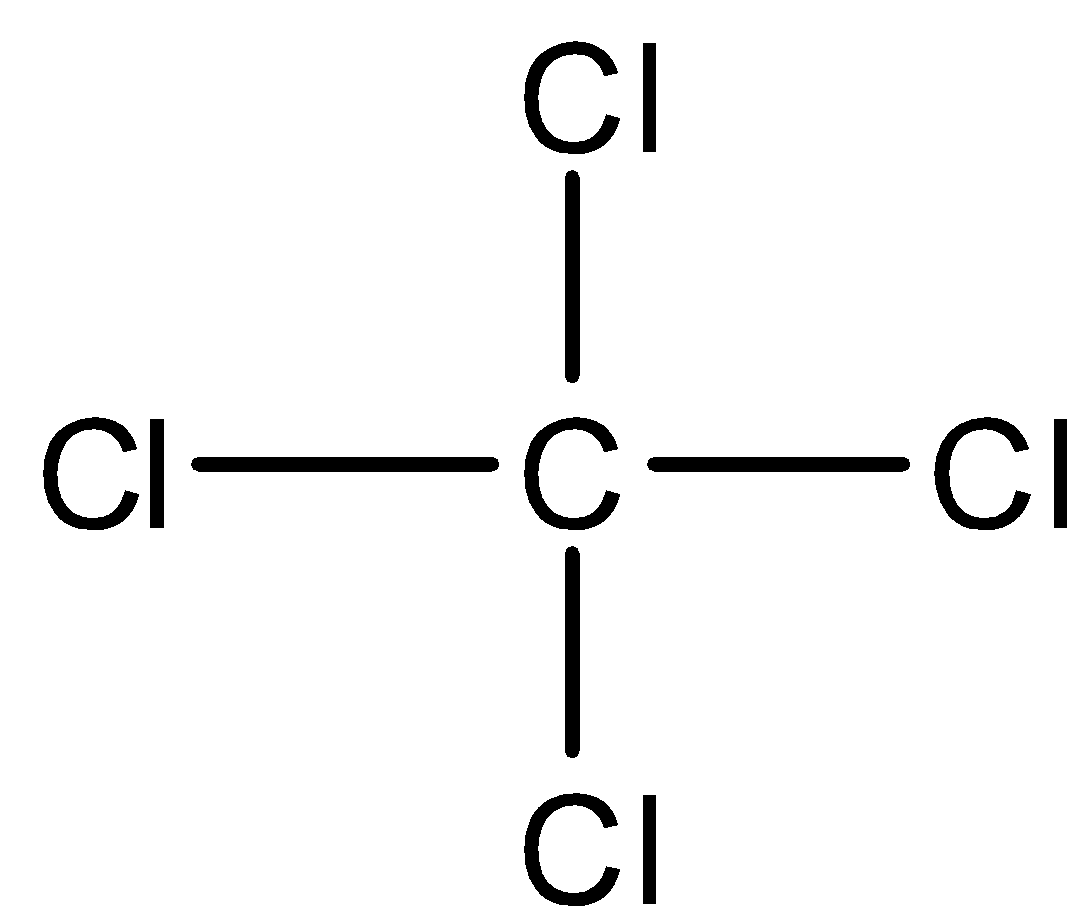
- Here, the four bonds are the symmetrical ones and are extended in all directions and therefore, the dipole moments of each chlorine atom cancel each other which makes them non polar in nature and thus have zero dipole moment.
- The polar molecule results from the unequal sharing of electrons which are the valence electrons and in the molecules like carbon tetrachloride these bonds are evenly distributed and cancel out. Thus there is no net dipole moment and the compound is non polar in nature.
Note: Note that the C−Cl bond is quite polar in nature with carbon having partial positive charge and chlorine having partial negative charge where chlorine is electronegative than chlorine and thus the dipole arising each of four C−Cl bonds are identical in magnitude and they cancel out which makes it non polar.
