Question
Question: How can the following conversions be brought about? i.Acetic acid to methyl cyanide ii.Acetaldeh...
How can the following conversions be brought about?
i.Acetic acid to methyl cyanide
ii.Acetaldehyde to formaldehyde
iii.Nitrobenzene to 2,4,6 tribromoaniline
Solution
For converting one compound to another you require certain reagents. Some conversions may happen in one step also but some may take place in 2-3 steps. For these conversions knowledge of reactions taking place is important.
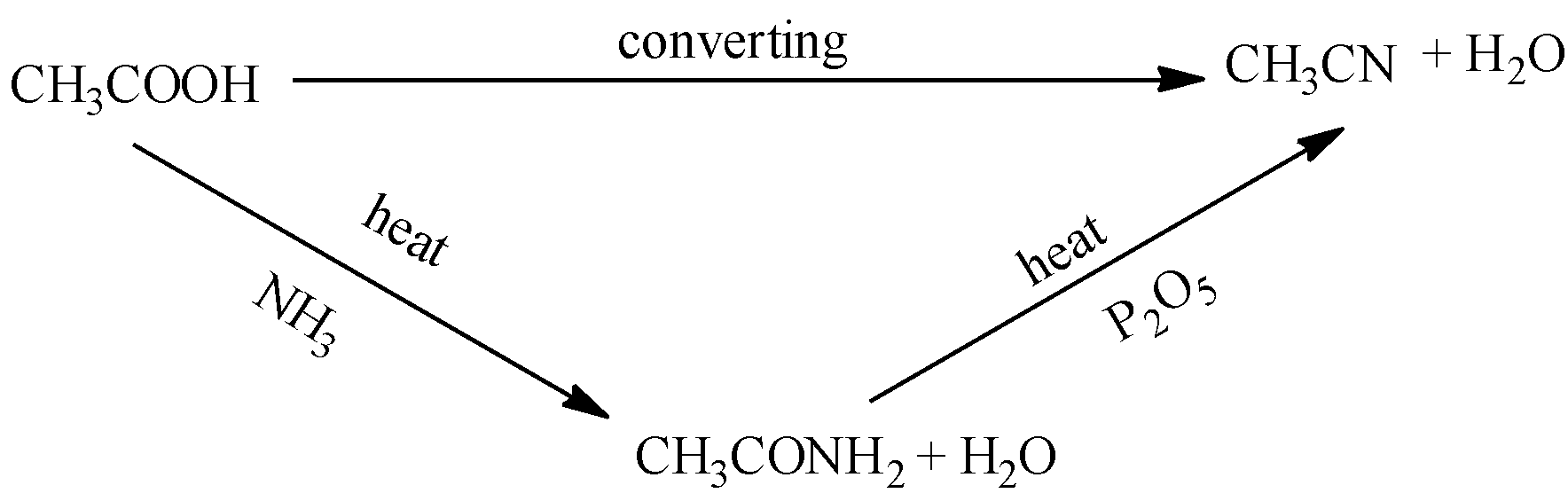
Complete answer: Let us first look at our first conversion. It says convert acetic acid to methyl cyanide. So first we will write the structures of both. So the structure of acetic acid is CH3COOHand of methyl cyanide is CH3CN. It can be seen that the reactant COOH group is changing into the CN group in the product and we know that there is no such reaction that will directly convert COOH to CN so we can say that the reaction is going to take place in more than one step. Hence below are the series of reaction for conversion of
i.Acetic acid to methyl cyanide.
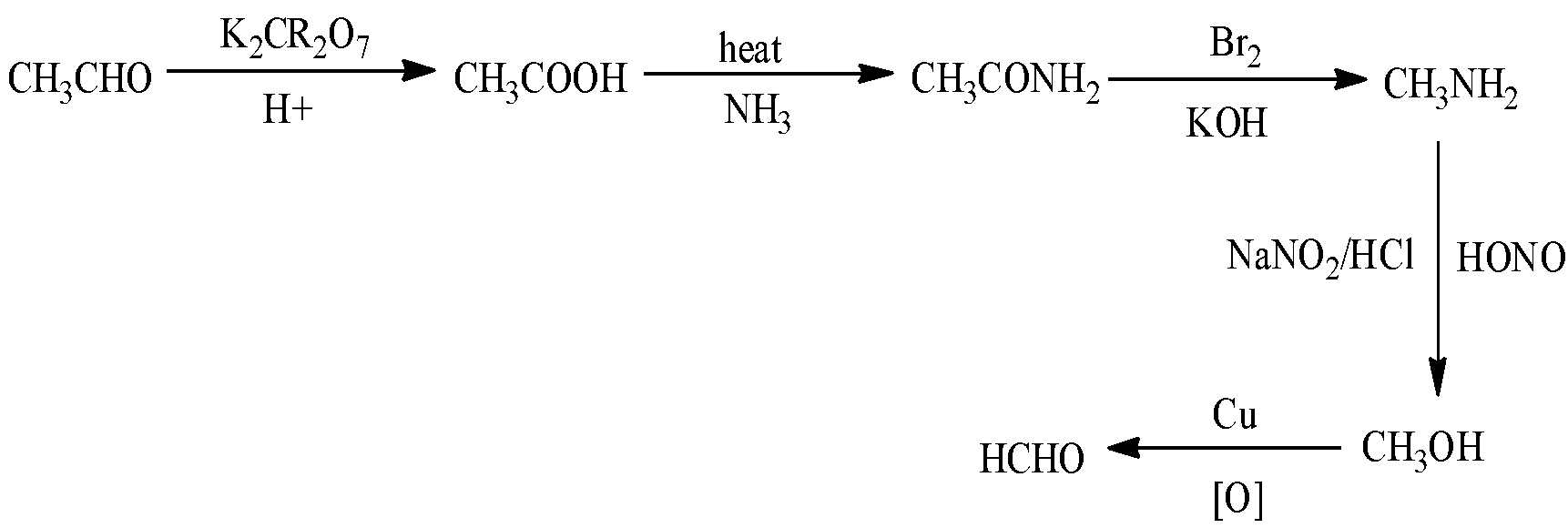
Now move on to our next conversion which is Acetaldehyde to formaldehyde. First, we will figure out their formula. Acetaldehyde has the formula CH3CHO and formaldehyde has the formula HCHO. Let us look into the reaction that will take place in this following conversion below:
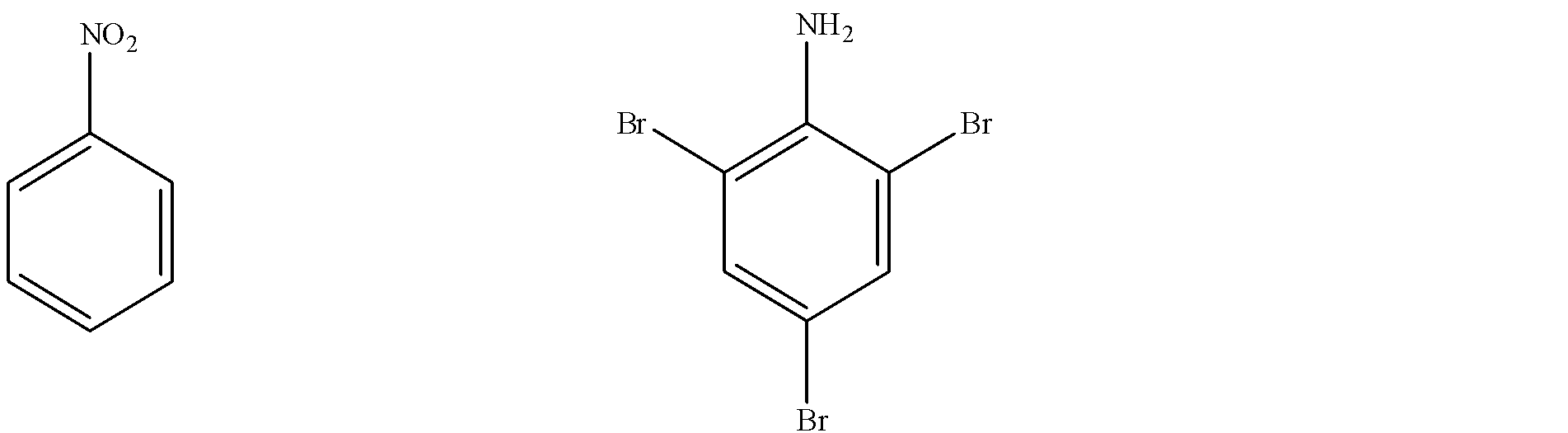
At last we have the third conversation to do which is Nitrobenzene to 2,4,6 tribromoaniline. The structure of nitrobenzene and of 2,4,6-tribromoaniline are:
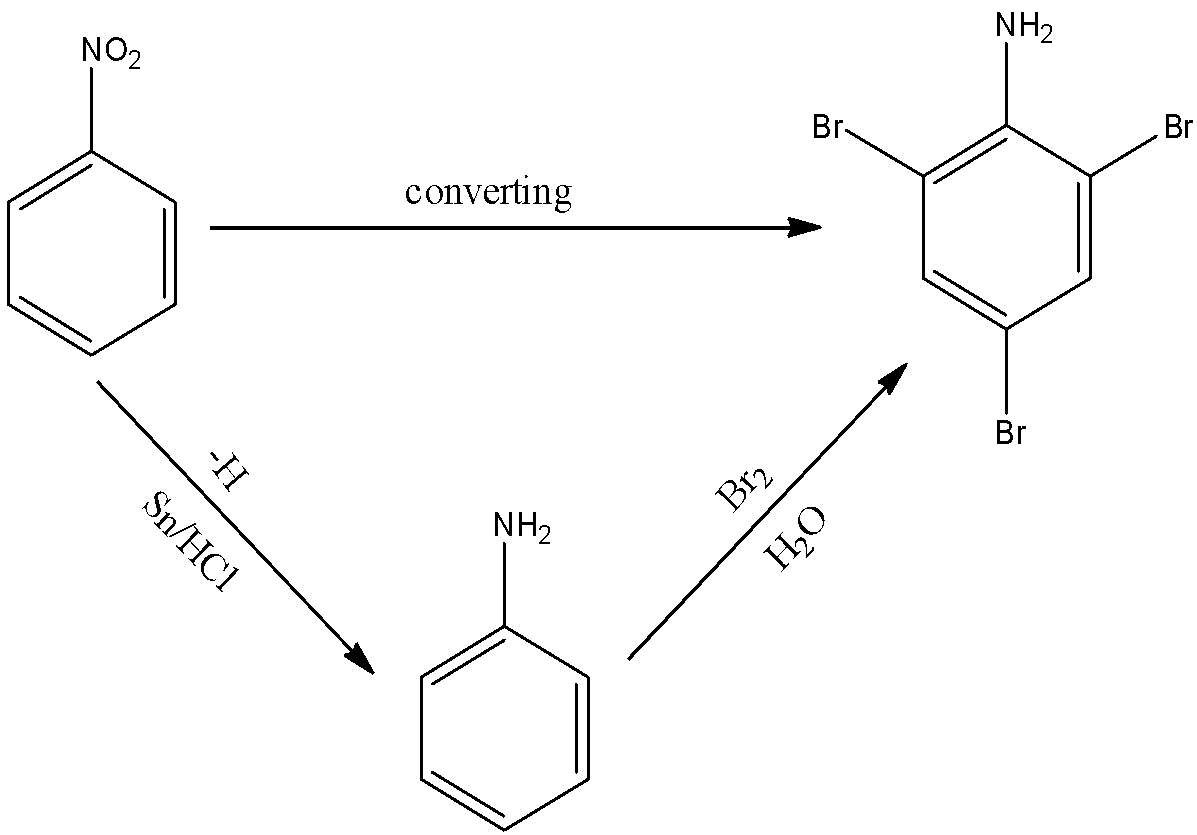
This conversion is just a two-step conversion. So the reactions taking place during the conversion are as follows:
Note:
For writing any conversions knowledge of reactions taking place is very important. As the only we may figure out how we will convert from one compound to the other. The conversions above are simple conversions. The only thing we need to remember is formulas for reagents. And one should also know the structures of the compound and their name.
