Question
Question: How can the following conversions be brought about: (A) Acetaldehyde to \({{propan - 2 - ol}}\). ...
How can the following conversions be brought about:
(A) Acetaldehyde to propan−2−ol.
(B) Nitro – benzene to p – aminoazobenzene.
(C) Acetic acid to methyl amine.
(D) Aniline to Benzene.
Solution
We should have prior knowledge of the functional groups and mechanism or reaction pathways through which conversions take place because if we don’t know the functional group properly, we cannot choose the right reagent for conversion.
Complete step by step answer:
(A) acetaldehyde is an aldehyde and we have to convert it in propane alcohol means addition of carbon is there with alcohol group so we can use Grignard reagent for the addition of carbon as well as the alcohol group. The conversion will be as follows:
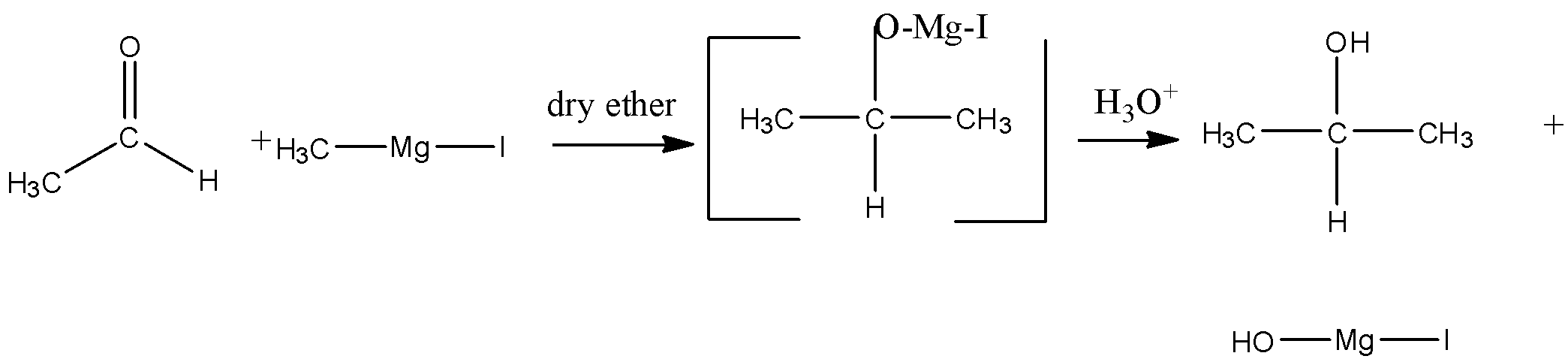
(B) Nitro benzene can be converted to amine by reduction by tin and HCl and then reacted with diazonium chloride salt of benzene which will give us p – aminoazobenzene. The reaction is as follows:
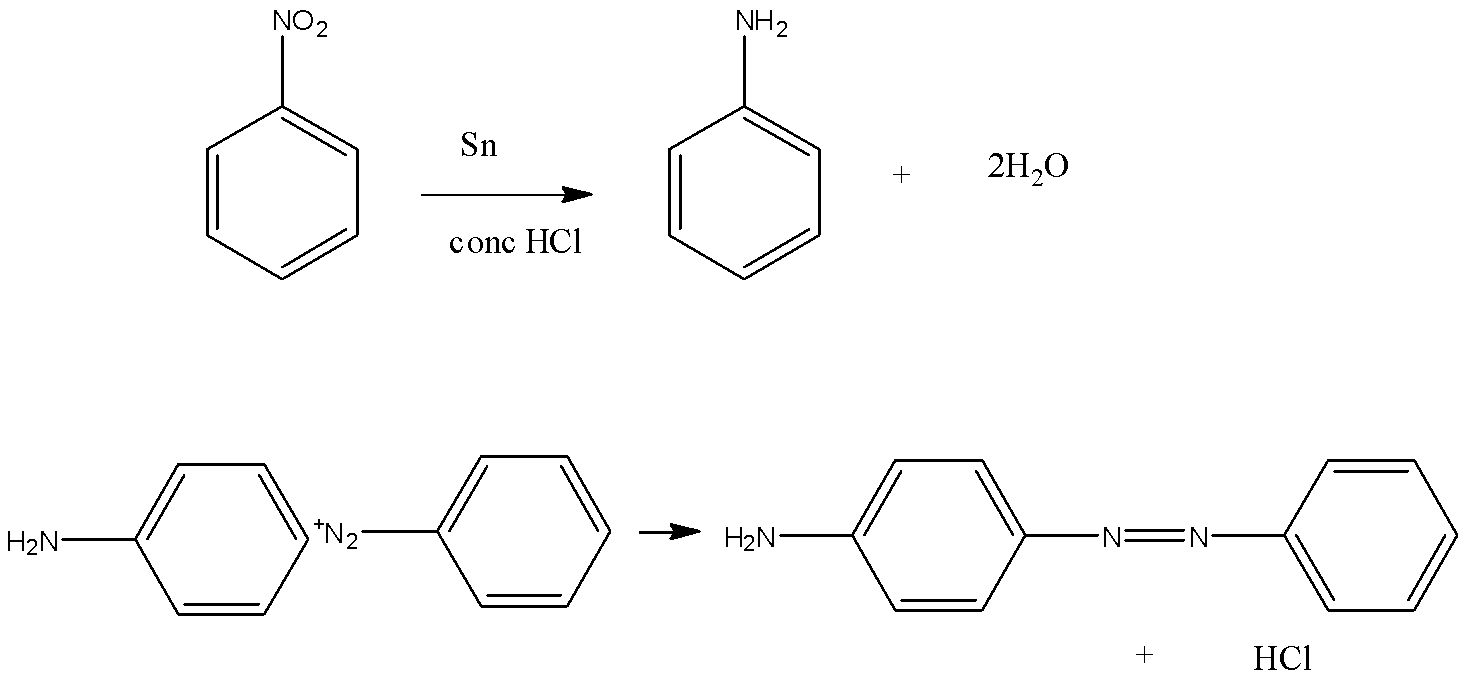
(C) Acetic acid is an acid with two carbon and we have to convert it in methyl amine which is one carbon compound so that means we have to deduct a carbon which can be possible by Hoffman bromamide reaction so we will convert acid into amide then into amine with less carbon. The reaction is:
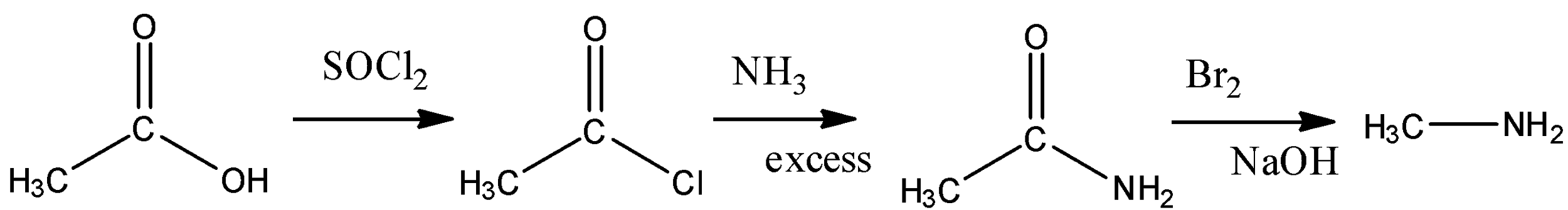
(D) Aniline is a benzene having an amine group attached to it , we have to convert it into benzene alone means we have to remove the amine group . We can convert it into diazonium salt and nitrogen becomes a good leaving group .
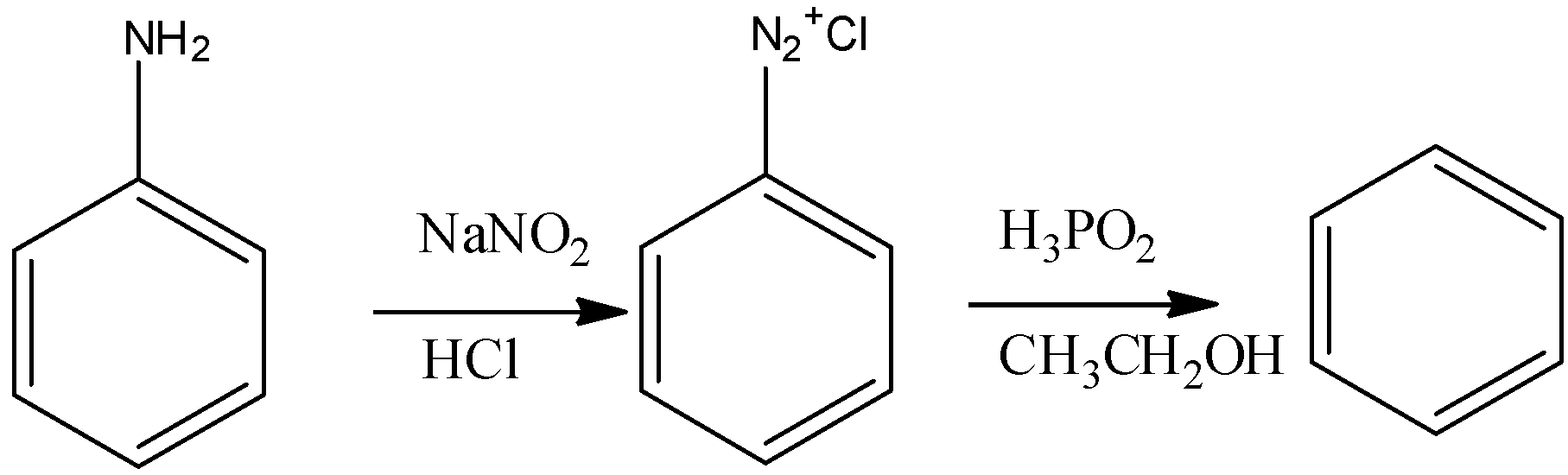
Note: With Grignard reagent we use only and only dry ether. We cannot use moist ether in this reaction as it contains water molecules and sodium and magnesium react violently with water so reaction will not be possible. The groups attached on benzene are not a good leaving group because of the resonance in the ring so for converting it to any type product the first step usually is to make the attached group a good leaving group.
