Question
Question: How can the conversion be carried out? Benzyl alcohol to 2-phenylethanoic acid....
How can the conversion be carried out?
Benzyl alcohol to 2-phenylethanoic acid.
Solution
Benzyl alcohol is an aromatic compound. It is having the molecular formula C6H5CH2OH. And 2-phenylethanoic acid is a carboxylic acid, with molecular formula C8H8ClO2 .
Complete step by step answer:
- We can see the conversion of Benzyl alcohol to 2-phenylethanoic acid:
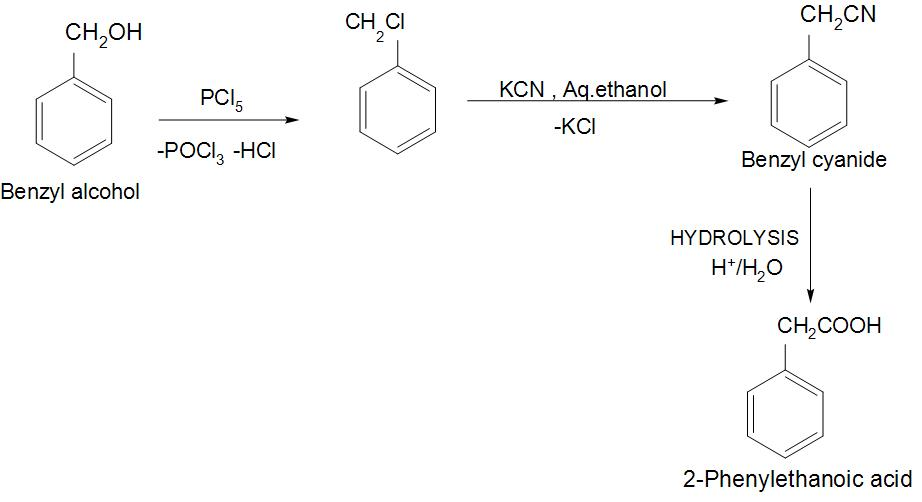
- We can see from the reaction that Benzyl alcohol is first reacted with PCl5 that is phosphorus pentachloride, to give benzyl chloride.
- Then in the next step, this benzyl chloride is reacted with KCN to give benzyl cyanide.
- Now, we need a carboxylic group instead of a cyanide group. So, we will bring about this change by subjecting this compound by the process of acid hydrolysis. And we will get 2-phenylethanoic acid as a product.
Additional Information:
- Benzyl alcohol is a colourless liquid which has pleasant aromatic odour.
- It is widely used, as it is having low vapour pressure, low polarity. It is also low toxicity.
- It is generally used as solvent for paints, waxes, inks etc. When it is applied to skin that is damaged or to the mucus membrane, then it is found that it acts as an antimicrobial agent.
- Benzyl alcohol is found to be used as a precursor to various ethers as well as esters. It is mainly used in the soap, and in various perfumes. For example, benzyl benzoate is widely used.
Note: Benzyl alcohol is used to produce many compounds. It is also found that it is also a precursor to many ethers. And it is miscible in alcohols and diethyl ether.
